I will be discussing this topic in various blog entries over the next several weeks, possibly longer, as I will concentrate on tropical systems affecting my area in other blogs, as warrented.
Sea level rise is one effect of anthropogenic global warming that is both complicated and simple. It is complicated by the complexity of ice sheet behavior. Some factors, such as ice sheet lubrication by meltwater penetrating to the interface between ice and rock beneath the ice sheets have only been known about for the past few years (more on this later), changes in the geoid as ice sheets melt and the mass balance of the Earth changes as a result, isostatic rebound continuing from the prior ice age, and from today's ice sheet melting. Also potential changes in weather patterns--shifts in mean high pressure centers and storm tracks, as well as in the ENSO cycle will probably be significant in augmenting or slowing sea level rise in many areas--unfortunately confident predictions of weather pattern shifts as anthropogenic global warming proceeds are not possible as yet.
Many laypeople assume that sea level rise will be uniform. After all if you add water to the ocean, it should rise everywhere, shouldn't it? Not so. The ice sheets of Greenland and Antarctica contain quadrillions of tons of mass, and have significant gravitational effects. If, for example, the Greenland Ice Sheet melted completely away tomorrow, global sea level would rise on average 23 feet. However, because gravitational attraction would be reduced near where the Greenland Ice sheet used to be, sea level would rise considerably less near Greenland, and considerably more at the antipode relative to Greenland. It could be that sea level rose 18 feet near Greenland, and 28 feet at Greenland's antipode. (Isostatic rebound of the land under the former Greenland Ice sheet would ultimately reduce this effect). Since the West Antarctic Ice sheet is near Greenland's antipode, and contains enough ice to raise sea levels by 16 feet, this gravitational effect would be mostly counterbalanced if it melted simultaneously, and most of the globe would experience sea level rises between 36 and 42 feet.
A good illustration of the non-uniformity of sea level rise is shown in the map below. It is perhaps unfortunate that sea level rise has been most marked in the western tropical Pacific while the North Atlantic and eastern Pacific which most of the developed world (North America and Europe) have seen sea level rises below the global average. A sea level rise of 10 mm (1 cm)/year on the northeast seaboard would concentrate minds! However, this map shows the ocean we have:
The simplicity of sea level rise is that it is inevitable. A warmer world is already resulting in temperate and tropical mountain glaciers melting everywhere (q.v.)
Mountain (alpine) glaciers do not have enough ice to raise sea levels by catastrophic amounts of course. However they do represent a significant fraction of the sea level rise occurring today. Another factor adding to sea level rise is thermal expansion as the oceans absorb heat trapped by rising concentrations of carbon dioxide, methane, and other greenhouse gases. Thermal expansion seems to be the primary driver of sea level rise at present. However, it is likely to be overwhelmed by massive melting in ice sheets during the late 21st and 22nd centuries. Recent work indicates that thermal expansion may not have played a large role in the Eemian interglacial 120,000 years ago compared to meltwater from ice sheets. However an extra few feet will just be another twist in the knife our descendants will have to face.
Sea level functions as a crude thermometer for the Earth. Sea level is determined by the temperature of the oceans, and the mass of the water the oceans contain. During the next three centuries, much of the more than 30 quadrillion tons of ice on our planet will melt. Even the East Antarctica Ice sheet. The amount of heat required to melt more than 30 quadrillion tons of ice is enormous. Enough to raise the atmospheric temperature to more than 1000 °F! While heat is absorbed by melting ice, global atmospheric and oceanic warming will be slowed. It will be much warmer, but temperatures will be livable in most places while the heat sink of ice melt operates. In 2250, St. Louis may have the average temperature of Phoenix or Miami, but it is perfectly possible to live in those cities (whether agriculture will be possible in the Midwest is another matter). The heat energy absorbed by melting ice and the warming of the oceans will both contribute to sea level rise, even as atmospheric temperatures do not rise by many tens or hundreds of degrees. The ocean depths, mostly between -4°C and 4°C, will also slowly absorb heat as it diffuses from above. (A warming of the ocean depths in future centuries, so that they were 30 °C all the way down would provoke an additional sea level rise of about 15 meters, as can be computed from this table but that won't happen as long as ice sheets exist to cool the ocean where they come in contact. In other words, not for many centuries.)
Is radiative forcing from our greenhouse gas emissions enough to remove all ice sheets from the surface of the earth?
Well let's see. Current radiative forcing by antropogenic greenhouse gas emissions is estimated to be 2.77 watts per square meter of the Earth in 2009. The surface area of the Earth is 510,072,000,000,000 square meters. This represents about 1,412,900,000,000,000 watts per second!
But wait! As MichaelSTL has reminded me, humankind has also increased aerosol emissions into the atmosphere, which reduce radiative forcing. He has generously provided the following two links, here and here.
So let's recalculate again. Using the figures for 2010 from this link courtesy of MichaelSTL, the net radiative forcing, adding the effect of aerosols, the figure of 2010 is 1.6628 W/m2. This revised figure gives us 850,000,000,000,000 watts per second for the whole surface of the Earth.
850 trillion watts, wow! But how much heat is that, really? After all, visiting Turkey in 1999, Mike and I were agog at the dollar being worth 650,000 Turkish lira, and spending 10 million Turkish lira for dinner!
Well it does collapse down. Going by this energy unit conversion table, a watt-hour (3600 watts) represents about .8598 of a kilocalorie, the heat required to increase the temperature of 1 kg of water 1 °C. 4187 watts raises one kg of water 1 °C. A kilogram of water is a liter, and there are 1,000 liters in a cubic meter, and a billion cubic meters in a cubic kilometer. Also, the melting of ice into water takes tremendous energy, as much as raising the temperature of water 80 °C! And ice is not necessarily at 0 °C--I'll assume that the average temperature of all the ice in ice sheets is -20 °C. The specific heat of ice is half that of water, so it takes 90 kcal to melt the typical kg of ice under that assumption.
So we have 850,000,000,000,000 watts per second to play with. Dividing by 4187 yields 203,000,000,000 kcal per second to play with. Dividing by 90 to melt a kilogram of ice yields enough energy to melt 2,255,000,000 kilograms of ice. Per second. This is 2,255,000 cubic meters of meltwater per second. Which is about 1 cubic kilometer every 7 minutes 23 1/3 seconds, or about 71,160 cubic kilometers of meltwater per year. Enough to melt all the ice on the surface of the Earth in 421 years. Of course this would mean no temperature increases in the atmosphere or oceans. All the heat would be absorbed by melting ice.
Of course we are not melting enough ice to yield 71,160 cubic kilometers of meltwater per year, even though our greenhouse gas emissions are trapping sufficient radiation to do so. The atmosphere and surface of the land is warming. The oceans are warming. The oceans are now our primary heat sink.
But the amount of anthropogenic greenhouse radiative forcing is increasing steadily. Current projections show it rising easily to 4, 5, and possibly 6 watts/square meter as the 21st century wears on. As temperatures rise, melting will accelerate on the margins of ice sheets, through the collapse of ice shelves in contact with warmer oceans, and acceleration of glacier movement provided by meltwater on the surface of glaciers and ice sheets working downwards to the ice/rock interface.
Rigorous scientific examination of the effects of anthropogenic global warming on sea level rise began surprisingly recently, with a paper by J. H. Mercer in 1978 on the possible collapse of the West Antarctic Ice Sheet. It would be very generous to say there was much scientific research into the effects of anthropogenic global warming on sea level in the 1960s and 1970s. If you had asked most qualified scientists back then, I suspect that they would have answered that increased snowfall on polar ice sheets would counteract the effects of melting alpine glaciers and thermal expansion. This 'consensus' was surprisingly long-lasting---after a few papers made a splash in the 1978-1981 period, research into the effects of AGW on sea levels, the hypothesis that AGW would result in major sea level rises fell into disfavor, or more accurately, neglect. This continued through the 1980s, and with some exceptions, through the 1990s. The discovery of outlet glacier acceleration in Greenland during the first years of the 2000s changed this. But that will be for a future blog entry.
Adding an interesting article about arctic sea ice during the Holocene Optimum.
Wednesday, August 3, 2011
Saturday, July 2, 2011
Global warming is here, and soon in an unprecedented way!
On June 30, NOAA released the new climate normals for the 1981-2010 period for the USA. Average temperatures were 0.5°F higher in 1981-2010 than in 1971-2000. Since the period 1981-2000 is included in both periods, this means that the 2000s were 1.5°F warmer than the 1970s in the USA! There can be no clearer indication that global warming is here!
This is a stunning rate of temperature rise. Half a degree per decade. If temperatures continue to rise at this rate, the 2090s will average 4.5°F warmer than the 2000s, and 6°F warmer than the 1970s! And the temperature rise will almost certainly not remain constant. Due to humanity's accelerating consumption of fossil fuels, carbon dioxide concentrations in the atmosphere will accelerate their rise and temperature rises will almost certainly accelerate as well. 0.5°F/decade almost certainly represents an underestimate of how much temperatures will increase during the 21st century.
Humanity has never lived in such a rapidly warming environment. Ever. Nothing comes close in our planet's history except for the Paleocene–Eocene Thermal Maximum (PETM)
The PETM was a huge warming triggered by mass releases of carbon dioxide and methane from the oceans 55.8 million years ago. Temperatures soared by more than 5°C from what was already a warmer environment than the present. The average temperature at the North Pole was 73°F, comparable to Miami. For tens of thousands of years there was not one snowflake. Not one floe of ice. No frost. Anywhere.
So how does our present addition of carbon dioxide to the atmosphere compare to what happened during the PETM? Much research has been done in the last decade, and the scope of carbon releases during the PETM, and their speed compared to our own time has become clear.
And the comparison is not good.
Here is a graph showing carbon releases into the atmosphere. Our current additions of carbon to the atmosphere are already more than 5 times greater than during the PETM, and are continuing to accelerate as the developing world industrializes, and the developed world does very little. Projections show that our carbon emissions will nearly triple, to 15 times the carbon emission rates of the PETM.
Graph of net carbon emissions into the atmosphere from Scientific American:
And a sediment core sample showing the PETM:
Dr. Lee Kump, one of the most renowned experts in the PETM, has written an article, The Last Great Global Warming for the July 2011 Scientific American. It makes sobering reading.
Some highlights from Dr. Kump's article:
Until recently, though, open questions about the event have made predictions speculative at best. New answers provide sobering clarity. They suggest the consequences of the planet’s last great global warming paled in comparison to what lies ahead, and they add new support for predictions that humanity will suffer if our course remains unaltered.
But what surprised us most was that this gas release was spread out over approximately 20,000 years—a time span between twice and 20 times as long as anyone has projected previously. That lengthy duration implies that the rate of injection during the PETM was less than two petagrams a year—a mere fraction of the rate at which the burning of fossil fuels is delivering greenhouse gases into the air today. Indeed, CO2 concentrations are rising probably 10 times faster now than they did during the PETM.
But what surprised us most was that this gas release was spread out over approximately 20,000 years—a time span between twice and 20 times as long as anyone has projected previously. That lengthy duration implies that the rate of injection during the PETM was less than two petagrams a year—a mere fraction of the rate at which the burning of fossil fuels is delivering greenhouse gases into the air today. Indeed, CO2 concentrations are rising probably 10 times faster now than they did during the PETM.
Species extinctions are on the rise, and shifting climate zones have already put surviving plants and animals on the move, often with the disease-bearing pests and other invasive species winning out in their new territories. Unlike those of the PETM, modern plants and animals now have roads, railways, dams, cities and towns blocking their migratory paths to more suitable climate. These days most large animals are already penned into tiny areas by surrounding habitat loss; their chances of moving to new latitudes to survive will in many cases be nil.
Current global warming is on a path to vastly exceed the PETM, but it may not be too late to avoid the calamity that awaits us. To do so requires immediate action by all the nations of the world to reduce the buildup of atmospheric carbon dioxide—and to ensure that the Paleocene-Eocene Thermal Maximum remains the last great global warming.
I have to disagree with Dr. Kump in the last extract from his article. Calamity does await us. The scientific community has known for almost 50 years that our fossil fuel emissions will warm the atmosphere. And yet next to nothing has been done. During the 1970s we had our best chance of limiting fossil fuel emissions during the first energy crisis. We made some cosmetic changes, but no real reforms. During the 1980s we slept through the soothing lullaby of the Reagan administration's neglect of environmental issues. During the 1990s Clinton triangulated away any meaningful environmental and energy reforms, and failed to provide any leadership on the Kyoto Protocol. The second Bush administration's hostility to environmental and energy reforms has been told in many long accounts, and requires no further comment by me.
And now we have Tea Party fanatics who would rather send the Earth straight to hell than acknowledge scientific reality, blocking any reforms to increase energy efficiency, or environmental protection. The Tea Party fanatics even want to reduce study and research about Global Warming!
We've never missed an opportunity to miss an opportunity.
I don't see how we can avoid calamity. It's not just the USA--cheap, carbon rich coal is powering the development of China--lip service is paid there to global warming, and greenwashing in China may be more prevalent than in any other country. India, South America, and even Africa are expanding their fossil fuel consumption rapidly. Coal is cheap, almost everywhere, and the worst thing we can consume. Short term thinking conquers all.
It makes me sad. I live on a beautiful barrier island, St. Simons Island with my partner. It is beautiful, a great place to grow up and a great place to live.
By 3000 CE, all of this will be gone. My house and island will be under a warm, acid sea, so deep that the sun will be only faintly visible. Rising to the surface, no land will be visible. The same will be true for land where billions of people live now, and where billions get their crops and foodstuffs from. And we're doing nothing to stop it. And with the current trajectory of carbon emissions, and our refusal to face the situation squarely, the remaking of our fair planet into an acidic, hot, steambath of a world seems inevitable.
This is a stunning rate of temperature rise. Half a degree per decade. If temperatures continue to rise at this rate, the 2090s will average 4.5°F warmer than the 2000s, and 6°F warmer than the 1970s! And the temperature rise will almost certainly not remain constant. Due to humanity's accelerating consumption of fossil fuels, carbon dioxide concentrations in the atmosphere will accelerate their rise and temperature rises will almost certainly accelerate as well. 0.5°F/decade almost certainly represents an underestimate of how much temperatures will increase during the 21st century.
Humanity has never lived in such a rapidly warming environment. Ever. Nothing comes close in our planet's history except for the Paleocene–Eocene Thermal Maximum (PETM)
The PETM was a huge warming triggered by mass releases of carbon dioxide and methane from the oceans 55.8 million years ago. Temperatures soared by more than 5°C from what was already a warmer environment than the present. The average temperature at the North Pole was 73°F, comparable to Miami. For tens of thousands of years there was not one snowflake. Not one floe of ice. No frost. Anywhere.
So how does our present addition of carbon dioxide to the atmosphere compare to what happened during the PETM? Much research has been done in the last decade, and the scope of carbon releases during the PETM, and their speed compared to our own time has become clear.
And the comparison is not good.
Here is a graph showing carbon releases into the atmosphere. Our current additions of carbon to the atmosphere are already more than 5 times greater than during the PETM, and are continuing to accelerate as the developing world industrializes, and the developed world does very little. Projections show that our carbon emissions will nearly triple, to 15 times the carbon emission rates of the PETM.
Graph of net carbon emissions into the atmosphere from Scientific American:
And a sediment core sample showing the PETM:
Dr. Lee Kump, one of the most renowned experts in the PETM, has written an article, The Last Great Global Warming for the July 2011 Scientific American. It makes sobering reading.
Some highlights from Dr. Kump's article:
Until recently, though, open questions about the event have made predictions speculative at best. New answers provide sobering clarity. They suggest the consequences of the planet’s last great global warming paled in comparison to what lies ahead, and they add new support for predictions that humanity will suffer if our course remains unaltered.
But what surprised us most was that this gas release was spread out over approximately 20,000 years—a time span between twice and 20 times as long as anyone has projected previously. That lengthy duration implies that the rate of injection during the PETM was less than two petagrams a year—a mere fraction of the rate at which the burning of fossil fuels is delivering greenhouse gases into the air today. Indeed, CO2 concentrations are rising probably 10 times faster now than they did during the PETM.
But what surprised us most was that this gas release was spread out over approximately 20,000 years—a time span between twice and 20 times as long as anyone has projected previously. That lengthy duration implies that the rate of injection during the PETM was less than two petagrams a year—a mere fraction of the rate at which the burning of fossil fuels is delivering greenhouse gases into the air today. Indeed, CO2 concentrations are rising probably 10 times faster now than they did during the PETM.
Species extinctions are on the rise, and shifting climate zones have already put surviving plants and animals on the move, often with the disease-bearing pests and other invasive species winning out in their new territories. Unlike those of the PETM, modern plants and animals now have roads, railways, dams, cities and towns blocking their migratory paths to more suitable climate. These days most large animals are already penned into tiny areas by surrounding habitat loss; their chances of moving to new latitudes to survive will in many cases be nil.
Current global warming is on a path to vastly exceed the PETM, but it may not be too late to avoid the calamity that awaits us. To do so requires immediate action by all the nations of the world to reduce the buildup of atmospheric carbon dioxide—and to ensure that the Paleocene-Eocene Thermal Maximum remains the last great global warming.
I have to disagree with Dr. Kump in the last extract from his article. Calamity does await us. The scientific community has known for almost 50 years that our fossil fuel emissions will warm the atmosphere. And yet next to nothing has been done. During the 1970s we had our best chance of limiting fossil fuel emissions during the first energy crisis. We made some cosmetic changes, but no real reforms. During the 1980s we slept through the soothing lullaby of the Reagan administration's neglect of environmental issues. During the 1990s Clinton triangulated away any meaningful environmental and energy reforms, and failed to provide any leadership on the Kyoto Protocol. The second Bush administration's hostility to environmental and energy reforms has been told in many long accounts, and requires no further comment by me.
And now we have Tea Party fanatics who would rather send the Earth straight to hell than acknowledge scientific reality, blocking any reforms to increase energy efficiency, or environmental protection. The Tea Party fanatics even want to reduce study and research about Global Warming!
We've never missed an opportunity to miss an opportunity.
I don't see how we can avoid calamity. It's not just the USA--cheap, carbon rich coal is powering the development of China--lip service is paid there to global warming, and greenwashing in China may be more prevalent than in any other country. India, South America, and even Africa are expanding their fossil fuel consumption rapidly. Coal is cheap, almost everywhere, and the worst thing we can consume. Short term thinking conquers all.
It makes me sad. I live on a beautiful barrier island, St. Simons Island with my partner. It is beautiful, a great place to grow up and a great place to live.
By 3000 CE, all of this will be gone. My house and island will be under a warm, acid sea, so deep that the sun will be only faintly visible. Rising to the surface, no land will be visible. The same will be true for land where billions of people live now, and where billions get their crops and foodstuffs from. And we're doing nothing to stop it. And with the current trajectory of carbon emissions, and our refusal to face the situation squarely, the remaking of our fair planet into an acidic, hot, steambath of a world seems inevitable.
Wednesday, June 15, 2011
New Maunder Minimum? Don't Count on it!
There's been a lot of loose talk in the past couple days about a new Maunder Minimum that will save us from the consequences of our greenhouse gas emissions. Would that were true. Even if a new Maunder Minimum does happen, the radiative forcing by additional carbon dioxide will overwhelm the effects of a reduction in solar activity, even a prolonged and deep one.
A NASA image of the Maunder Minimum:
Andrew Rivkin writes about this latest deus ex machina here.
Dr. Doug Biesecker, the head of NOAA's sunspot team, has created a slideshow presentation here.
And Dr. Biesecker has written up a report "Predicting Solar Cycle 25" which goes into further detail.
An article about the case for a second Maunder Minimum, from The Economist, a source I generally find credible, is here.
Richard Black of the BBC also has an interesting take on the possibility of a Maunder Minimum II and its effects here.
The main issue is that even if a new Maunder Minimum does occur, it will offset only a small part of the radiative forcing of the additional carbon dioxide in the atmosphere. Estimates of the reduction of solar radiation during the Maunder Minimum are on the order of 1 watt/square meter. But the radiative absorption by carbon dioxide and other greenhouse gases is already almost 2 watts/square meter, and will be around 9 watts/square meter by 2100. A Maunder Minimum II would slow global warming slightly, but not stop it.
I hope Maunder Minimum II does take place. It would be helpful. And give us some breathing room for enacting reforms in energy consumption and protecting the environment in ways to slow down global warming further.
Unfortunately, our political and business history shows that even if a new Maunder Minimum takes place, we will squander the opportunity and declare the problem solved. Humanity has never faced the global warming problem squarely in the past, and I hardly expect it will do so now. And when the sun resumed its normal radiative output, global warming will quickly become catastrophic.
Tuesday, June 14, 2011
Atmospheric Carbon Dioxide hits new record; the rise's acceleration.
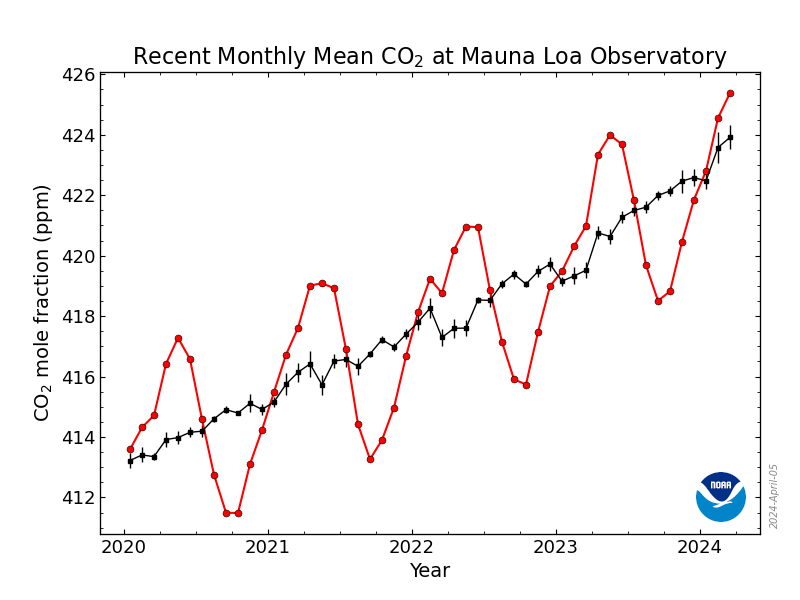
Atmospheric carbon dioxide hit a new all time record at the Mauna Loa observation site, as it has in every May since observations began. 394.15 ppm, on track to hit 400 ppm in spring 2014. Recent graph below:
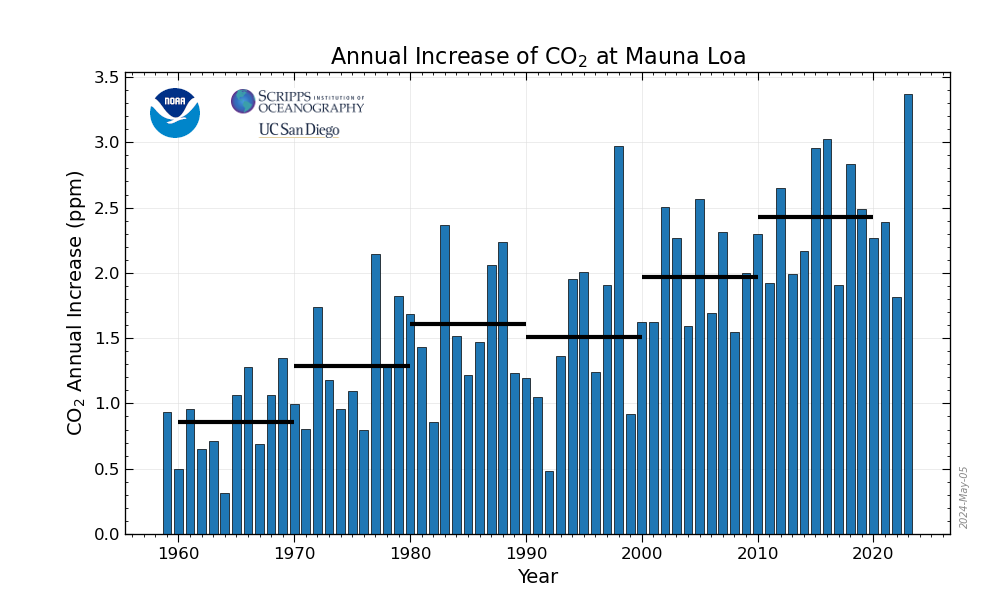
Carbon dioxide's rate of increase in the atmosphere has increased during every decade of record, except for the 1990s, when it was slowed by the effects of Mount Pinatubo's eruption in 1991. The 2000s show a continuing acceleration, making up for the slowdown of the 1990s. The rate of acceleration is about 0.3 ppm faster for each year per decade. It is very disturbing.
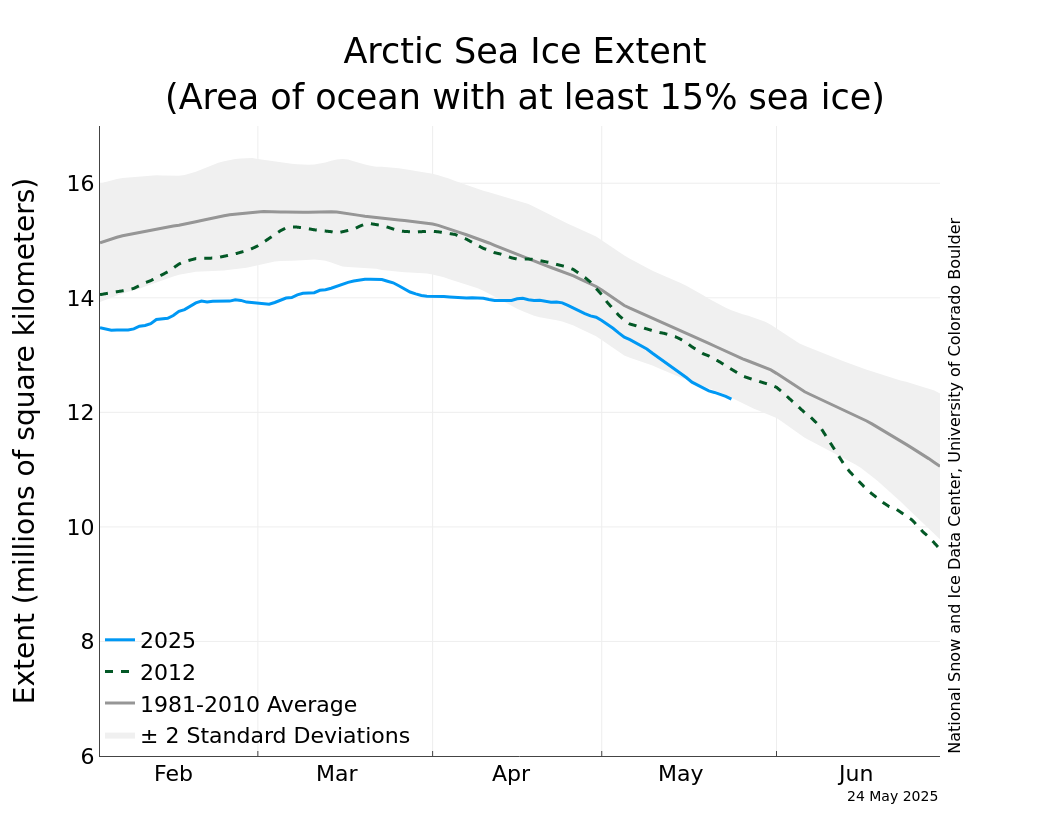
Finally, arctic sea ice is melting very rapidly. It is too early to say that it will reach a new record lowest summer minimum this September, but it is behaving as it would were it to reach a new minimum this year.
And I love Mike Luckovich's cartoons. Especially this one:
Carbon dioxide's rate of increase in the atmosphere has increased during every decade of record, except for the 1990s, when it was slowed by the effects of Mount Pinatubo's eruption in 1991. The 2000s show a continuing acceleration, making up for the slowdown of the 1990s. The rate of acceleration is about 0.3 ppm faster for each year per decade. It is very disturbing.
Finally, arctic sea ice is melting very rapidly. It is too early to say that it will reach a new record lowest summer minimum this September, but it is behaving as it would were it to reach a new minimum this year.
And I love Mike Luckovich's cartoons. Especially this one:
Saturday, June 11, 2011
Global warming since 1995 'now significant'
A story with powerful conclusions in updated data--global warming is REAL. And the trend since 1995 is undeniable (except by the simple-minded and dishonest).
The story, with links to relevant reports included:
The story, with links to relevant reports included:
Monday, May 30, 2011
The Causes of Climate Change conference--Boulder, CO 1965
Are human technology and activities forces of geophysical scope, capable of affecting the entire planet Earth? Surely not, thought most earth scientists in 1940. But a quarter century later, the consensus was beginning to shift. Several factors were involved in this shift. First of all, unprecedented economic growth. As I noted previously, during the first half of the 20th century, continuous, exponential economic growth was not a given. Two world wars and the Great Depression had interrupted economic growth in many developed countries. In 1950, industrial output was lower than 1913 in several major economic powers, such as Germany, France, and Japan. The Soviet Union and the United Kingdom were not much better. True, the USA had more than tripled its industrial production during that period.
Many scientists in the 1940s and 1950s assumed that carbon dioxide emissions would remain relatively constant. Gilbert Plass assumed that humankind's carbon dioxide emissions would be a flat 6 billion tons annually. (The IEA released a report on May 30, 2011 that humankind's carbon dioxide emissions soared past 30 billion tons for the first time in 2010, q.v.).
By 1965, humankind's carbon dioxide emissions were greater than 12 billion tons annually, and rising by more than half a billion tons per year. The assumption that carbon dioxide emissions would remain relatively low was incorrect.
Second, the work of Drs. Roger Revelle and Gilbert Plass showed that the oceans would not, could not, absorb all of humankind's carbon dioxide emissions, and that additional carbon dioxide would increase absorption of infrared radiation.
And then, Dr. Charles Keeling proved through his meticulous measurements of atmospheric carbon dioxide, and his isotopic analysis, that humankind's activities were increasing carbon dioxide in the atmosphere. During the first couple years of his measurements, it was postulated by some scientists that there could be a natural cycle that causes carbon dioxide concentrations to fluctuate, and it was possible that he was observing the uptrend of a natural cycle. And in fact there is such a natural cycle---the ENSO cycle does cause carbon dioxide concentrations to fluctuate by a few parts per million. But as carbon dioxide concentrations continued to rise each year, by 1962/1963 there was no possible doubt. Atmospheric carbon dioxide concentrations were rising, and humankind was responsible. For the past 50 years, no serious scientist has doubted that.
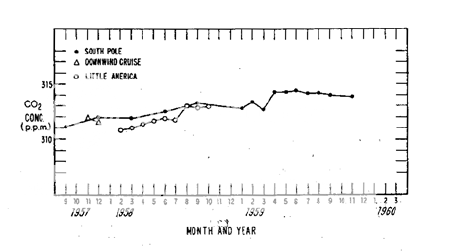
This graph shows the Keeling measurements for atmospheric carbon dioxide from 1958-1966. I would have preferred a 1958-1965 graph to dovetail with what the scientists at the "Causes of Climate Change" conference knew, but it is close enough for my blog, and the trend was clear:
Although the conference was organized by Dr. Revelle, the inspiration for it happened in 1963 Dr. Revelle had a conversation with astrophysicist and atmospheric physicist Dr. Walter Orr Robertsm who founded the National Center for Atmospheric Research in 1960. Dr. Roberts pointed out the aircraft contrails in the sky early one morning, and said that they would be indistinguishable from natural cirrus clouds in a few hours. They had a morning meeting, and when it broke for lunch, Dr. Revelle and Dr. Roberts went outside and could see the contrails from earlier, smearing out. By the time they finished lunch, the contrails looked just like cirrus clouds. Dr. Roberts wondered if adding cirrus clouds to the atmosphere could change the climate. Dr. Revelle wondered too.
The National Center for Atmospheric Research. The futuristic buildings served as a set for the comedy classic Sleeper, directed and written by Woody Allen.
Also in 1963, Dr. Ried Bryson (1920-2008), meteorologist and geologist, and one of the few scientific opponents to anthropogenic global warming, noticed on a flight across India to a scientific conference noticed that although the sky was cloudless, he could not see the ground, with all the smoke from brush and cooking fires. He noticed similar hazes in Brazil and sub-Saharan Africa. Dr. Bryson thought that global dimming would trigger global cooling--and that was the major threat that humankind's activities would have on the environment, a view that influenced Dr. Isaac Asimov (1920-1992) during the 1960s and 1970s.
During the last few years of his life, Dr. Bryson revised his views and concluded that global warming from the greenhouse gases humankind emits are the greater threat.
An aside on global dimming. It is a legitimate scientific viewpoint, and in fact during the 1950s and 1960s, the rise in global temperatures did pause, and increased pollution in the industrialized countries coupled with increases in tropical haze from cooking fires and brush fires and fires set to clear forest land may have had enough of an impact to blunt the rise in global temperature. Since the 1970s, increased pollution controls in the most advanced countries coupled with the relentless rise in concentrations of atmospheric carbon dioxide have clearly overwhelmed any cooling effect from aerosols in the atmosphere which promote global cooling.
To save time and effort, I am not going to go into every scientist that attended the conference, or go into everyone's theories or what they said. The main purpose of the Boulder conference, at least officially, was to discuss the mechanisms of natural climate change.
Until the 1950s, it had been believed that there were four major ice ages over the past 2 million years. And this viewpoint persisted in most of the general scientific community until the 1970s. In fact, the four ice ages are referred to in Dr. Arthur C. Clarke's novel 2001: A Space Odyssey (1968). [There are not many references to the novel, which was released in July 1968. There are many references to the film, of course.] This went with the reassuring uniformitarian mindset that typified earth science studies from the time of geologist Dr. James Hutton to the mid 20th century. Over the past 50 years, the realization that changes in the Earth's environment can be sudden and far reaching has led to a more neo-catastrophism mindset, of which the extinction of the dinosaurs by the impact of a comet/asteroid is the most prominent example.
Discoveries in the 1950s lead to the realization that ice ages and interglacials were far more frequent--more than 20 glaciations were identified by 1965, although this new knowledge took a long time to diffuse into the general scientific community. This work had been done by Drs. Harold Urey and Cesare Emiliani (q.v.) Their discoveries also indicated that climate change could have been rapid, although this discovery was resisted. However, in the early 1960s, work by Dr. Wallace Smith Broecker (Wally) (1931-) on ancient tropical corals also showed evidence that climate could change rapidly. [Dr. Broecker will be the subject of a forthcoming blog entry.] Also, Dr. Edward Lorenz (1917-2008) discussed his work on computer simulations of weather patterns, which was proving to be chaotic. Dr. Lorenz wondered whether climate states could also prove to be chaotic.
The implications were becoming clear. Climate had changed more rapidly in the past than had been believed before. Most of the scientists who attended the Boulder Conference on Climate Change were convinced of that by the time the conference was over. But it took a long time for this new consensus to diffuse into the general scientific community. To use an analogy, the discoveries of the 1950s had planted the seed of the possibility of rapid climate change. The 1965 conference was when the seed sprouted.
The work by Dr. Charles Keeling (q.v.) had shown definitively by 1965 that humankind's activities were measurably and significantly increasing the amount of atmospheric carbon dioxide. Dr. Gilbert Plass had overthrown the old belief that increases in atmospheric carbon dioxide would not increase the amount of infrared radiation trapped by the atmosphere---additional atmospheric carbon dioxide clearly would. So would humankind's carbon dioxide emissions trigger a sudden change in the Earth's climate? That question left the attendees of the Boulder conference uneasy.
The minutes of the conference published in 1966 contain this interesting statement: "We are just now beginning to realize that the atmosphere is not a dump of unlimited capacity but we do not yet know what the atmosphere's capacity is"*
*National Academy of Sciences, Committee on Atmospheric Sciences Panel on Weather and Climate Modification, Weather and Climate Modification: Problems and Prospects. 2 vols. (Washington, D.C., National Academy of Sciences, 1966), col. 1, p. 10.
Many scientists in the 1940s and 1950s assumed that carbon dioxide emissions would remain relatively constant. Gilbert Plass assumed that humankind's carbon dioxide emissions would be a flat 6 billion tons annually. (The IEA released a report on May 30, 2011 that humankind's carbon dioxide emissions soared past 30 billion tons for the first time in 2010, q.v.).
By 1965, humankind's carbon dioxide emissions were greater than 12 billion tons annually, and rising by more than half a billion tons per year. The assumption that carbon dioxide emissions would remain relatively low was incorrect.
Second, the work of Drs. Roger Revelle and Gilbert Plass showed that the oceans would not, could not, absorb all of humankind's carbon dioxide emissions, and that additional carbon dioxide would increase absorption of infrared radiation.
And then, Dr. Charles Keeling proved through his meticulous measurements of atmospheric carbon dioxide, and his isotopic analysis, that humankind's activities were increasing carbon dioxide in the atmosphere. During the first couple years of his measurements, it was postulated by some scientists that there could be a natural cycle that causes carbon dioxide concentrations to fluctuate, and it was possible that he was observing the uptrend of a natural cycle. And in fact there is such a natural cycle---the ENSO cycle does cause carbon dioxide concentrations to fluctuate by a few parts per million. But as carbon dioxide concentrations continued to rise each year, by 1962/1963 there was no possible doubt. Atmospheric carbon dioxide concentrations were rising, and humankind was responsible. For the past 50 years, no serious scientist has doubted that.
This graph shows the Keeling measurements for atmospheric carbon dioxide from 1958-1966. I would have preferred a 1958-1965 graph to dovetail with what the scientists at the "Causes of Climate Change" conference knew, but it is close enough for my blog, and the trend was clear:
Although the conference was organized by Dr. Revelle, the inspiration for it happened in 1963 Dr. Revelle had a conversation with astrophysicist and atmospheric physicist Dr. Walter Orr Robertsm who founded the National Center for Atmospheric Research in 1960. Dr. Roberts pointed out the aircraft contrails in the sky early one morning, and said that they would be indistinguishable from natural cirrus clouds in a few hours. They had a morning meeting, and when it broke for lunch, Dr. Revelle and Dr. Roberts went outside and could see the contrails from earlier, smearing out. By the time they finished lunch, the contrails looked just like cirrus clouds. Dr. Roberts wondered if adding cirrus clouds to the atmosphere could change the climate. Dr. Revelle wondered too.
The National Center for Atmospheric Research. The futuristic buildings served as a set for the comedy classic Sleeper, directed and written by Woody Allen.
Also in 1963, Dr. Ried Bryson (1920-2008), meteorologist and geologist, and one of the few scientific opponents to anthropogenic global warming, noticed on a flight across India to a scientific conference noticed that although the sky was cloudless, he could not see the ground, with all the smoke from brush and cooking fires. He noticed similar hazes in Brazil and sub-Saharan Africa. Dr. Bryson thought that global dimming would trigger global cooling--and that was the major threat that humankind's activities would have on the environment, a view that influenced Dr. Isaac Asimov (1920-1992) during the 1960s and 1970s.
During the last few years of his life, Dr. Bryson revised his views and concluded that global warming from the greenhouse gases humankind emits are the greater threat.
An aside on global dimming. It is a legitimate scientific viewpoint, and in fact during the 1950s and 1960s, the rise in global temperatures did pause, and increased pollution in the industrialized countries coupled with increases in tropical haze from cooking fires and brush fires and fires set to clear forest land may have had enough of an impact to blunt the rise in global temperature. Since the 1970s, increased pollution controls in the most advanced countries coupled with the relentless rise in concentrations of atmospheric carbon dioxide have clearly overwhelmed any cooling effect from aerosols in the atmosphere which promote global cooling.
To save time and effort, I am not going to go into every scientist that attended the conference, or go into everyone's theories or what they said. The main purpose of the Boulder conference, at least officially, was to discuss the mechanisms of natural climate change.
Until the 1950s, it had been believed that there were four major ice ages over the past 2 million years. And this viewpoint persisted in most of the general scientific community until the 1970s. In fact, the four ice ages are referred to in Dr. Arthur C. Clarke's novel 2001: A Space Odyssey (1968). [There are not many references to the novel, which was released in July 1968. There are many references to the film, of course.] This went with the reassuring uniformitarian mindset that typified earth science studies from the time of geologist Dr. James Hutton to the mid 20th century. Over the past 50 years, the realization that changes in the Earth's environment can be sudden and far reaching has led to a more neo-catastrophism mindset, of which the extinction of the dinosaurs by the impact of a comet/asteroid is the most prominent example.
Discoveries in the 1950s lead to the realization that ice ages and interglacials were far more frequent--more than 20 glaciations were identified by 1965, although this new knowledge took a long time to diffuse into the general scientific community. This work had been done by Drs. Harold Urey and Cesare Emiliani (q.v.) Their discoveries also indicated that climate change could have been rapid, although this discovery was resisted. However, in the early 1960s, work by Dr. Wallace Smith Broecker (Wally) (1931-) on ancient tropical corals also showed evidence that climate could change rapidly. [Dr. Broecker will be the subject of a forthcoming blog entry.] Also, Dr. Edward Lorenz (1917-2008) discussed his work on computer simulations of weather patterns, which was proving to be chaotic. Dr. Lorenz wondered whether climate states could also prove to be chaotic.
The implications were becoming clear. Climate had changed more rapidly in the past than had been believed before. Most of the scientists who attended the Boulder Conference on Climate Change were convinced of that by the time the conference was over. But it took a long time for this new consensus to diffuse into the general scientific community. To use an analogy, the discoveries of the 1950s had planted the seed of the possibility of rapid climate change. The 1965 conference was when the seed sprouted.
The work by Dr. Charles Keeling (q.v.) had shown definitively by 1965 that humankind's activities were measurably and significantly increasing the amount of atmospheric carbon dioxide. Dr. Gilbert Plass had overthrown the old belief that increases in atmospheric carbon dioxide would not increase the amount of infrared radiation trapped by the atmosphere---additional atmospheric carbon dioxide clearly would. So would humankind's carbon dioxide emissions trigger a sudden change in the Earth's climate? That question left the attendees of the Boulder conference uneasy.
The minutes of the conference published in 1966 contain this interesting statement: "We are just now beginning to realize that the atmosphere is not a dump of unlimited capacity but we do not yet know what the atmosphere's capacity is"*
*National Academy of Sciences, Committee on Atmospheric Sciences Panel on Weather and Climate Modification, Weather and Climate Modification: Problems and Prospects. 2 vols. (Washington, D.C., National Academy of Sciences, 1966), col. 1, p. 10.
Wednesday, May 25, 2011
Charles David Keeling
Dr. Charles David Keeling (1928-2005) was the giant of the 20th century in atmospheric carbon dioxide studies. It was his single-mindedness that established continuous carbon dioxide monitoring. Without him, it might have been decades more before continuous carbon dioxide monitoring was established.
Dr. Charles Keeling was born in Scranton, PA on April 20, 1928. A precocious child, he obtained his B.S. in chemistry from the University of Illinois in 1948 at age 20, and earned his PhD in chemistry from Northwestern University in 1954.
Dr. Keeling had many interests---he was an accomplished piano player and loved hiking and camping in the mountains of California, when he moved he moved after obtaining his doctorate. He was a postdoctorate fellow in geochemistry at the California Institute of Technology from 1954-1956, where he developed new instruments which for the first time could measure carbon dioxide in the atmosphere in parts per billion. His instruments were later supplanted by the electron capture dectector invented by Dr. James Lovelock in 1957, which was adopted worldwide for sampling in the 1960s.
In 1956 he was invited to join the Scripps Institution of Oceanography by Dr. Roger Revelle (q.v.)
Dr. Revelle said about Dr. Keeling "He's a peculiar guy. He wants to measure CO2 in his belly...and he wants to measure it with the greatest precision and the greatest accuracy he possibly can.". Keeling had taken his instruments to sites in the Sierra mountains, but there were problems. When the wind shifted so that the sites were downwind of major cities like San Francisco and Sacramento, the concentrations rose sharply. What Dr. Keeling needed was a pristine site, thousands of miles away from large cities and industrial concentrations.
The 1950s and 1960s were a golden age for scientific research. The impetus of the Cold War, and unprecedented prosperity and rising wealth stimulated large and increasing research budgets. The International Geophysical Year of 1957-1958 (IGY) further augmented research budgets. Climate change, much less anthropogenic global warming, was not a big priority with the IGY, but Dr. Revelle made funds available for Dr. Keeling to make his carbon dioxide observations at the Mauna Loa Observatory, beginning March 1, 1958. Dr. Keeling also supervised a carbon dioxide sampling program from the new Antarctic bases established during the IGY.
Mauna Loa was an ideal site for Dr. Keeling's measurements. It was far from any population concentration, and the site being over 11,000' in elevation placed it above the inversion in the atmosphere that separates the low level moist trade winds from the middle levels of the atmosphere, reducing anthropogenic influences even further.
Continuous carbon dioxide monitoring was a new idea. Before discussing it with Dr. Keeling, Dr. Revelle had envisioned sampling carbon dioxide at various pristine sites around the world during the IGY, and then a new sample program comparing the IGY readings to observations made during a subsequent sampling program ~ 20 years later, say in 1980. And the Antarctic observations were dropped in the year or two after the IGY. Scientific research budgets were large and rising, but not unlimited, and atmospheric carbon dioxide measurements were not the highest priority. And as we shall see, there were serious threats to cut off the Mauna Loa measurements in the 1960s, before the importance of the measurements was fully appreciated by the scientific community.
Dr. Keelings measurements soon showed that carbon dioxide was accumulating in the atmosphere. Dr. Revelle had been proven correct--the buffer mechanism he had proposed that prevented the oceans from absorbing all the CO2 humankind was emitting was making a measurable difference in atmospheric concentrations!
Dr. Keeling published his preliminary findings in the June 1960 of Tellus in the article "The Concentration and isotopic abundances of carbon dioxide in the atmosphere" This article contains the graph I embedded below:
Two years wasn't much though. After all, there could be some sort of atmospheric cycle going on. Today we know that is ridiculous, and we can safely dismiss the denier cranks who make that argument, but 50 years ago it was still a reasonable position. Dr. Revelle continued funding Dr. Keeling's carbon dioxide measurement program, but outside events intervened. A stock market 'crash' in the spring of 1962 wiped out more than a quarter of stocks' value---the market soon recovered, but there was a disruption to the Scripps Institution's endowment. Also in the early 1960s there was a sort of pause in the growth of budgets for scientific research, and increasing amounts were being absorbed by NASA. There were waves of growth in scientific research funding in the late 1950s and the mid 1960s, but the early 1960s saw something of a pause. And most important of all, no research agency considered Dr. Keeling's carbon dioxide measurements truly compelling---the measurements were interesting, yes, but not enough for an agency or institution to fund themselves. And the Mauna Loa Observatory was relatively isolated---an advantage in obtaining pristine atmospheric carbon dioxide measurements---but a disadvantage in that it was expensive to supply and operate.
Dr. Revelle was able to divert some funding to keep Dr. Keeling's measurement program going through 1963, and by late in that year had some promising indications of permanent funding from the National Science Foundation. (NSF) But in January 1964 the money ran out. Carbon dioxide measurements at Mauna Loa Observatory stopped.
This triggered a reaction in the scientific community--Dr Keeling's carbon dioxide series was suddenly appreciated much more in its absence!--and the NSF quickly approved permanent funding. After a 3 month hiatus in February, March, and April 1964, the Mauna Loa measurement program was resumed on May 1, 1964, and has continued to the present day.
As I said before, some scientists looked at the first 2 years of data from the Antarctic stations and Mauna Loa with legitimately skeptical eyes. The ENSO cycle was not well known 50 years ago (which does affect carbon dioxide concentrations in the atmosphere, particularly in the Pacific), but a cycle was plausible. However, as the measurement program went on, and carbon dioxide continued to increase its concentration in the atmosphere every year, such skepticism, never widely held, fell by the wayside. Since the mid 1960s, no reputable meteorologist, climate scientist, or physicist has denied that humankind's emissions are driving the atmospheric carbon dioxide increase. By the mid 1960s, the increase was undeniable. The following graph shows how carbon dioxide concentrations were increasing through the mid 1960s.
Note the funding hiatus in 1964. Mind the gap!
The importance of Dr. Keeling's measurements of atmospheric carbon dioxide cannot be overstated. Dr. Revelle showed that the oceans would not absorb all the carbon dioxide humankind emitted. Dr. Plass proved that increases in the concentration of carbon dioxide in the atmosphere would increase infrared radiation absorption. And Dr. Keeling proved that carbon dioxide concentrations were increasing, in a measurable and significant amount. As these facts disseminated through the scientific community, the scientific consensus swung decisively to the reality of anthropogenic global warming by the mid 1960s, and has remained so.
An aside here---it is frequently asserted by deniers that meteorologists and climate scientists believed in global cooling in the 1970s. This is utterly false. An analysis of peer-reviewed articles on future climate change from the period 1965-1979 shows that predictions of anthropogenic global warming outnumber predictions of anthropogenic global cooling by more than 6 to 1 (specifically 44 to 7).
Whenever a denier claims that the scientific community was predicting global cooling in the 1970s, that denier is either ignorant, or deliberately lying.
Dr. Keeling was concerned enough about rising carbon dioxide levels to participate in a panel by the Conservation Foundation on March 12, 1963 "Implications of Rising Carbon Dioxide Content of the Atmosphere", the report issued being among the first to speculate that anthropogenic global warming could be dangerous to the Earth's biological and environmental systems. It includes on page 6: "many life forms would be annihilated" [in the tropics] if emissions continued unchecked in the upcoming centuries. They also projected that carbon dioxide emissions could raise the average surface temperature of the earth by as much as 4°C during the next century (1963-2063)
Rising concern was also brought forth in 1965 when the President's Science Advisory Committee formed a panel to address environmental issues, including a climate change sub-panel. The 1965 meeting and report of this panel will be the subject of a future blog entry.
Dr. Keeling did have a monomania concerning carbon dioxide, but it was a productive monomania. Dr. Keeling was made professor of oceanography at the Scripps Institute in 1968, and received many honors for his scientific work. A short list of some of the honors he received:
Second Half Century Award of the American Meteorological Society, 1981
Maurice Ewing Medal of the American Geophysical Union, 1991
Blue Planet Prize from the Science Council of Japan and the Asahi Foundation, 1993
National Medal of Science, by George W. Bush in 2002
Tyler Prize for Environmental Achievement in 2005 (shared with Lonnie Thompson)
Dr. Keeling married Louise Barthold in 1955, and they had 5 children. One of whom, Dr. Ralph Keeling, is a climatologist at the Scripps Institute himself, following in his father's footsteps. Dr. Ralph Keeling is the current director of the Scripps CO2 Program.
Dr. Keeling was a lifelong Republican, of a type we don't see much of anymore--a Republican with a strong concern for the environment and science. Dr. Keeling deeply regretted and was disappointed by the politicization of science, and the abandonment of science by the large parts of the Republican party during the last two decades of his life. When ideology and scientific fact conflict, it should be the ideology that changes--because the facts will not. Dr. Keeling continued his measurements of carbon dioxide until he died of a heart attack on June 20, 2005.
A picture of Dr. Charles Keeling in 1997:
Here is the latest Keeling Curve, with the full record of carbon dioxide levels in the atmosphere:
A report released today (May 30, 2011) by the IEA reports that our CO2 emissions reached a new record in 2010, 30.6 billion tons. CO2 emissions in 2010 were 5% higher than the previous record in 2008.
Dr. Charles Keeling was born in Scranton, PA on April 20, 1928. A precocious child, he obtained his B.S. in chemistry from the University of Illinois in 1948 at age 20, and earned his PhD in chemistry from Northwestern University in 1954.
Dr. Keeling had many interests---he was an accomplished piano player and loved hiking and camping in the mountains of California, when he moved he moved after obtaining his doctorate. He was a postdoctorate fellow in geochemistry at the California Institute of Technology from 1954-1956, where he developed new instruments which for the first time could measure carbon dioxide in the atmosphere in parts per billion. His instruments were later supplanted by the electron capture dectector invented by Dr. James Lovelock in 1957, which was adopted worldwide for sampling in the 1960s.
In 1956 he was invited to join the Scripps Institution of Oceanography by Dr. Roger Revelle (q.v.)
Dr. Revelle said about Dr. Keeling "He's a peculiar guy. He wants to measure CO2 in his belly...and he wants to measure it with the greatest precision and the greatest accuracy he possibly can.". Keeling had taken his instruments to sites in the Sierra mountains, but there were problems. When the wind shifted so that the sites were downwind of major cities like San Francisco and Sacramento, the concentrations rose sharply. What Dr. Keeling needed was a pristine site, thousands of miles away from large cities and industrial concentrations.
The 1950s and 1960s were a golden age for scientific research. The impetus of the Cold War, and unprecedented prosperity and rising wealth stimulated large and increasing research budgets. The International Geophysical Year of 1957-1958 (IGY) further augmented research budgets. Climate change, much less anthropogenic global warming, was not a big priority with the IGY, but Dr. Revelle made funds available for Dr. Keeling to make his carbon dioxide observations at the Mauna Loa Observatory, beginning March 1, 1958. Dr. Keeling also supervised a carbon dioxide sampling program from the new Antarctic bases established during the IGY.
Mauna Loa was an ideal site for Dr. Keeling's measurements. It was far from any population concentration, and the site being over 11,000' in elevation placed it above the inversion in the atmosphere that separates the low level moist trade winds from the middle levels of the atmosphere, reducing anthropogenic influences even further.
Continuous carbon dioxide monitoring was a new idea. Before discussing it with Dr. Keeling, Dr. Revelle had envisioned sampling carbon dioxide at various pristine sites around the world during the IGY, and then a new sample program comparing the IGY readings to observations made during a subsequent sampling program ~ 20 years later, say in 1980. And the Antarctic observations were dropped in the year or two after the IGY. Scientific research budgets were large and rising, but not unlimited, and atmospheric carbon dioxide measurements were not the highest priority. And as we shall see, there were serious threats to cut off the Mauna Loa measurements in the 1960s, before the importance of the measurements was fully appreciated by the scientific community.
Dr. Keelings measurements soon showed that carbon dioxide was accumulating in the atmosphere. Dr. Revelle had been proven correct--the buffer mechanism he had proposed that prevented the oceans from absorbing all the CO2 humankind was emitting was making a measurable difference in atmospheric concentrations!
Dr. Keeling published his preliminary findings in the June 1960 of Tellus in the article "The Concentration and isotopic abundances of carbon dioxide in the atmosphere" This article contains the graph I embedded below:
Two years wasn't much though. After all, there could be some sort of atmospheric cycle going on. Today we know that is ridiculous, and we can safely dismiss the denier cranks who make that argument, but 50 years ago it was still a reasonable position. Dr. Revelle continued funding Dr. Keeling's carbon dioxide measurement program, but outside events intervened. A stock market 'crash' in the spring of 1962 wiped out more than a quarter of stocks' value---the market soon recovered, but there was a disruption to the Scripps Institution's endowment. Also in the early 1960s there was a sort of pause in the growth of budgets for scientific research, and increasing amounts were being absorbed by NASA. There were waves of growth in scientific research funding in the late 1950s and the mid 1960s, but the early 1960s saw something of a pause. And most important of all, no research agency considered Dr. Keeling's carbon dioxide measurements truly compelling---the measurements were interesting, yes, but not enough for an agency or institution to fund themselves. And the Mauna Loa Observatory was relatively isolated---an advantage in obtaining pristine atmospheric carbon dioxide measurements---but a disadvantage in that it was expensive to supply and operate.
Dr. Revelle was able to divert some funding to keep Dr. Keeling's measurement program going through 1963, and by late in that year had some promising indications of permanent funding from the National Science Foundation. (NSF) But in January 1964 the money ran out. Carbon dioxide measurements at Mauna Loa Observatory stopped.
This triggered a reaction in the scientific community--Dr Keeling's carbon dioxide series was suddenly appreciated much more in its absence!--and the NSF quickly approved permanent funding. After a 3 month hiatus in February, March, and April 1964, the Mauna Loa measurement program was resumed on May 1, 1964, and has continued to the present day.
As I said before, some scientists looked at the first 2 years of data from the Antarctic stations and Mauna Loa with legitimately skeptical eyes. The ENSO cycle was not well known 50 years ago (which does affect carbon dioxide concentrations in the atmosphere, particularly in the Pacific), but a cycle was plausible. However, as the measurement program went on, and carbon dioxide continued to increase its concentration in the atmosphere every year, such skepticism, never widely held, fell by the wayside. Since the mid 1960s, no reputable meteorologist, climate scientist, or physicist has denied that humankind's emissions are driving the atmospheric carbon dioxide increase. By the mid 1960s, the increase was undeniable. The following graph shows how carbon dioxide concentrations were increasing through the mid 1960s.
Note the funding hiatus in 1964. Mind the gap!
The importance of Dr. Keeling's measurements of atmospheric carbon dioxide cannot be overstated. Dr. Revelle showed that the oceans would not absorb all the carbon dioxide humankind emitted. Dr. Plass proved that increases in the concentration of carbon dioxide in the atmosphere would increase infrared radiation absorption. And Dr. Keeling proved that carbon dioxide concentrations were increasing, in a measurable and significant amount. As these facts disseminated through the scientific community, the scientific consensus swung decisively to the reality of anthropogenic global warming by the mid 1960s, and has remained so.
An aside here---it is frequently asserted by deniers that meteorologists and climate scientists believed in global cooling in the 1970s. This is utterly false. An analysis of peer-reviewed articles on future climate change from the period 1965-1979 shows that predictions of anthropogenic global warming outnumber predictions of anthropogenic global cooling by more than 6 to 1 (specifically 44 to 7).
Whenever a denier claims that the scientific community was predicting global cooling in the 1970s, that denier is either ignorant, or deliberately lying.
Dr. Keeling was concerned enough about rising carbon dioxide levels to participate in a panel by the Conservation Foundation on March 12, 1963 "Implications of Rising Carbon Dioxide Content of the Atmosphere", the report issued being among the first to speculate that anthropogenic global warming could be dangerous to the Earth's biological and environmental systems. It includes on page 6: "many life forms would be annihilated" [in the tropics] if emissions continued unchecked in the upcoming centuries. They also projected that carbon dioxide emissions could raise the average surface temperature of the earth by as much as 4°C during the next century (1963-2063)
Rising concern was also brought forth in 1965 when the President's Science Advisory Committee formed a panel to address environmental issues, including a climate change sub-panel. The 1965 meeting and report of this panel will be the subject of a future blog entry.
Dr. Keeling did have a monomania concerning carbon dioxide, but it was a productive monomania. Dr. Keeling was made professor of oceanography at the Scripps Institute in 1968, and received many honors for his scientific work. A short list of some of the honors he received:
Second Half Century Award of the American Meteorological Society, 1981
Maurice Ewing Medal of the American Geophysical Union, 1991
Blue Planet Prize from the Science Council of Japan and the Asahi Foundation, 1993
National Medal of Science, by George W. Bush in 2002
Tyler Prize for Environmental Achievement in 2005 (shared with Lonnie Thompson)
Dr. Keeling married Louise Barthold in 1955, and they had 5 children. One of whom, Dr. Ralph Keeling, is a climatologist at the Scripps Institute himself, following in his father's footsteps. Dr. Ralph Keeling is the current director of the Scripps CO2 Program.
Dr. Keeling was a lifelong Republican, of a type we don't see much of anymore--a Republican with a strong concern for the environment and science. Dr. Keeling deeply regretted and was disappointed by the politicization of science, and the abandonment of science by the large parts of the Republican party during the last two decades of his life. When ideology and scientific fact conflict, it should be the ideology that changes--because the facts will not. Dr. Keeling continued his measurements of carbon dioxide until he died of a heart attack on June 20, 2005.
A picture of Dr. Charles Keeling in 1997:
Here is the latest Keeling Curve, with the full record of carbon dioxide levels in the atmosphere:
A report released today (May 30, 2011) by the IEA reports that our CO2 emissions reached a new record in 2010, 30.6 billion tons. CO2 emissions in 2010 were 5% higher than the previous record in 2008.
Tuesday, May 17, 2011
A striking image of arctic sea ice concentrations in 2007
I thought this deserved it's own entry. I wonder how this year will shape up? Arctic sea ice has been falling very rapidly so far in May, but it's too early to say if arctic sea ice will reach a record low this year.
Plus a couple of news stories I found interesting:
Murky exoplanet 'could host life'
Human arrival 'wiped out' Hawaii's unique crabs
Plus a couple of news stories I found interesting:
Murky exoplanet 'could host life'
Human arrival 'wiped out' Hawaii's unique crabs
Friday, May 13, 2011
Gilbert Norman Plass
Dr. Gilbert Norman Plass (1920/21/22-2004) was the last scientist before Charles Keeling to make important contributions to the study of global warming. He was a Canadian physicist, who obtained his PhD at John Hopkins, and not a climatologist or meteorologist. But it was the publication of his insight into the the reality that increases in carbon dioxide in the atmosphere would increase infrared radiation absorption and global surface temperatures, along with Roger Revelle's work on the oceanic chemistry of carbon dioxide, and Charles Keeling's measurements proving that carbon dioxide was increasing in the atmosphere that established the scientific consensus that humankind's activities could and would warm the climate of the Earth.
Back at the end of the 19th century, Svante Arrhenius made his famous proposal of anthropogenic global warming. But although a few lonely scientists believed him and carried on research in anthropogenic global warming, Knut Johan Ångström carried out experiments in laboratory conditions that appeared to show that carbon dioxide was saturated as an infrared absorber. These experiments were done near sea level, with the higher temperatures and humidity of sea level air. Dr. Plass wondered about the absorption of infrared radiation by carbon dioxide at greater altitudes in the atmosphere, and what increases in carbon dioxide would mean.
A huge hint that Ångström's experiments with carbon dioxide's infrared absorption were not correct had been noted as early as 1890---and yet was ignored. Frank Washington Very and Samuel Pierpont Langley had carried out infrared astronomy for the moon beginning in 1890, and noted that more infrared radiation from the moon was observed when it was near its zenith than when it was near the horizon. These observations proved that carbon dioxide was not saturated in terms of absorbing infrared radiation--it it were, then the absorption of infrared radiation would be the same no matter what altitude above the horizon the moon was. Amazingly, Arrhenius and all climate scientists seemed to have remained unaware of Very and Langley's work for more than 60 years!
Gilbert Plass was either born in1 1920, 1921 or 1922 (my sources disagree) in Toronto and quickly showed strong aptitudes for math and science. After scoring a 168 on an IQ test and having it confirmed, he was allowed to skip years in HS and the government of Canada paid for his education at Harvard where he graduated with a BS in physics in 1941, and earned his doctorate in physics from Princeton in 1947.
After World War II, as part of the United States' rapidly expanding scientific research, the Office of Naval Research. Much of this research was esoteric---who knew what kind of scientific discoveries were to be made, and what impact they could have! The 30 years after World War II were a time when government institutions and Bell Labs supported pure scientific research, and allowed research scientists to follow their own muses, unlike today's more commercial research climate (no pun intended).
The Office of Naval Research was interested in absorption of infrared radiation in the atmosphere as it related to heat-seeking missiles and other weaponry. Beginning in the late 1940s, observations at the Thule (now named Qaanaaq) base in the northwest part of Greenland suggested strongly that variations in carbon dioxide strongly changed absorption of infrared radiation by carbon dioxide. Dr. Plass was a physicist, not a climatologist or meteorologist. However, he was aware of the scientific consensus that carbon dioxide was saturated as an infrared radiation absorber. What if this was not the case?
Dr. Plass was curious about this, and worked on his own time to see if carbon dioxide was really saturated as an infrared absorber. From observations at arctic bases and at high altitude flights were missile tests were conducted, he concluded that it was not. But concluding this was one thing, proving it was another.
In 1953 Dr. Plass moved from Canada to southern California to work with Lockheed on missile testing and guidance. And for the first time he had access to a computer. As a competent physicist, Dr. Plass knew how to craft programs to analyze the absorption of infrared radiation by carbon dioxide using quantum mechanics. Without a computer, he would never have been able to make the calculations. Dr. Plass felt confident enough in his belief that our carbon dioxide emissions would warm the Earth's climate that in 1953 he contributed to an article in Time magazine saying so. But the computers of the 1950s were balky and slow, and he had to do his research on his own time. So it took him more than 2 years to mathematically prove that infrared absorption was not saturated at current levels of carbon dioxide in the atmosphere.
Dr. Plass published his work in the July 1956 issue of American Scientist. Dr. Plass made some errors that oddly enough, cancelled each other out. Dr. Plass underestimated the amount of carbon dioxide humankind was emitting into the atmosphere---he gave a figure of 6 billion tons. We now know it was 8.8 billion tons in 1956. Dr. Plass also overestimated the radiative forcing of additional carbon dioxide in the atmosphere. Dr. Plass estimated that a doubling of carbon dioxide in the atmosphere would yield a radiative forcing of 8.3 watts per square meter under clear conditions, and of 5.8 watts per square meter under cloudy conditions. He only had observational from a few arctic bases and brief airborne tests piggybacking on missile testing. The explosion in the earth sciences generated by the 1957-1958 International Geophysical Year, in which high altitude observations were made in the Andes and Antarctica refined this to 4 watts per square meter, under both clear and cloudy conditions. These refinements came quickly---by 1960 all atmospheric physicists knew that a doubling of carbon dioxide would have the correct, 4 watts per square meter warming. And anyone who still goes by the Ångström experiments of 1901-1902 can be dismissed as an ignorant quack (it is amazing how much Ångström's experiments are still cited by deniers).
Dr. Plass's paper is summarized and discussed here.
Dr. Plass made several simplifying assumptions. He assumed no change in water vapor, and no change in absorption of carbon dioxide by the oceans---as I said he was not a climatologist or meteorologist, or oceanographer. He simply ignored feedbacks in his paper. He also assumed that humankind's carbon dioxide emissions into the atmosphere would remain constant at 6 billion tons per year (and as we know, it was already 8.8 billion tons in 1956.
Dr. Plass made some of these simplifying assumptions because of 'known unknowns'--he knew he was not qualified to assume how water vapor and other feedbacks would behave. Also, in the 1950s, while computers were beginning to be used in science, their power was extremely limited by today's standards.
The assumption that carbon dioxide emissions would remain constant seem more inexplicable. As I have discussed in previous blog entries, in the 1950s long-term economic growth on a planet-wide scale was not a given. Countries such as France, Germany, and Japan had lower industrial output in 1950 than in 1913. The United Kingdom and the Soviet Union were not much better. Yes by the mid 1950s the industrial output of the United States was more than triple its 1913 level, and so were Canada's and Australia's. But among those three, only the United States was emitting carbon dioxide at a globally significant level.
This seems amazing, when we consider than global carbon dioxide emissions more than doubled over the next 15 years. But scientists had no way to know that was going to happen--wars had set back economic growth on a generational scale twice in the recent past, and there was no reason to suppose that would not happen again.
Dr. Plass concluded that carbon dioxide could double over a century and raise global temperatures 1.5° C over the next century, a figure that agrees closely with the definitive Charney report of 1979, which gives a 1.2 °C figure. Dr. Plass also concluded that known reserves of carbon-based fossil fuels would add enough CO2 to the atmosphere to warm the surface of the Earth by 7 °C (12.6 °F) by 3000 CE. At such a planetary average surface temperature, the Greenland and West Antarctic ice sheets would be gone, and the East Antarctic ice sheet would be going.
I must repeat here that Dr. Plass was not a climatologist or meteorologist. He did not try to compute feedbacks such as decreasing albedo or increased water vapor in the atmosphere. He focused on the radiative properties of CO2 only.
During the 1960s, returning to his work on CO2 and the Earth's climate, he concluded that net feedbacks were positive, and that each doubling of CO2 in the atmosphere would increase surface temperatures by 3.6 °C.
It has to be said that Dr. Plass did not optimally research and craft his meteorology papers. His lack of some knowledge of meteorology led him to some errors---he tried to compute atmospheric properties and constants that had been solved by others, sometimes decades previously. And he made some mistakes. Today's research on climate feedbacks produce much larger increases in surface temperature.
But I must also repeat that Dr. Plass's proof that increased CO2 in the atmosphere increases infrared radiation absorption did hold. No meteorologist or climatologist denies that now. Not reputable ones.
Roger Revelle had shown how oceanic chemistry buffers prevent the oceans from absorbing all our carbon dioxide emissions. Plass had proven that carbon dioxide was not saturated in the atmosphere from an infrared radiation absorption standpoint. But were human activities really causing carbon dioxide to accumulate in the atmosphere? That question still remained.
And Charles Keeling was to definitively answer it. But that's for the next blog entry.
Here is a picture of Dr. Gilbert Plass:
Dr. Plass left Lockheed in 1960 to join the research staff of Ford's aeronautical division. Dr. Plass also edited Infrared Physics and Technology, a peer-reviewed scientific publication. Dr. Plass worked there until 1963, when he accepted a position as first professor of atmospheric and space sciences with the University of Texas at Arlington, where he remained for 5 years. In 1968 he joined the faculty of Texas A&M University, ultimately becoming head of the department of physics.
Back at the end of the 19th century, Svante Arrhenius made his famous proposal of anthropogenic global warming. But although a few lonely scientists believed him and carried on research in anthropogenic global warming, Knut Johan Ångström carried out experiments in laboratory conditions that appeared to show that carbon dioxide was saturated as an infrared absorber. These experiments were done near sea level, with the higher temperatures and humidity of sea level air. Dr. Plass wondered about the absorption of infrared radiation by carbon dioxide at greater altitudes in the atmosphere, and what increases in carbon dioxide would mean.
A huge hint that Ångström's experiments with carbon dioxide's infrared absorption were not correct had been noted as early as 1890---and yet was ignored. Frank Washington Very and Samuel Pierpont Langley had carried out infrared astronomy for the moon beginning in 1890, and noted that more infrared radiation from the moon was observed when it was near its zenith than when it was near the horizon. These observations proved that carbon dioxide was not saturated in terms of absorbing infrared radiation--it it were, then the absorption of infrared radiation would be the same no matter what altitude above the horizon the moon was. Amazingly, Arrhenius and all climate scientists seemed to have remained unaware of Very and Langley's work for more than 60 years!
Gilbert Plass was either born in1 1920, 1921 or 1922 (my sources disagree) in Toronto and quickly showed strong aptitudes for math and science. After scoring a 168 on an IQ test and having it confirmed, he was allowed to skip years in HS and the government of Canada paid for his education at Harvard where he graduated with a BS in physics in 1941, and earned his doctorate in physics from Princeton in 1947.
After World War II, as part of the United States' rapidly expanding scientific research, the Office of Naval Research. Much of this research was esoteric---who knew what kind of scientific discoveries were to be made, and what impact they could have! The 30 years after World War II were a time when government institutions and Bell Labs supported pure scientific research, and allowed research scientists to follow their own muses, unlike today's more commercial research climate (no pun intended).
The Office of Naval Research was interested in absorption of infrared radiation in the atmosphere as it related to heat-seeking missiles and other weaponry. Beginning in the late 1940s, observations at the Thule (now named Qaanaaq) base in the northwest part of Greenland suggested strongly that variations in carbon dioxide strongly changed absorption of infrared radiation by carbon dioxide. Dr. Plass was a physicist, not a climatologist or meteorologist. However, he was aware of the scientific consensus that carbon dioxide was saturated as an infrared radiation absorber. What if this was not the case?
Dr. Plass was curious about this, and worked on his own time to see if carbon dioxide was really saturated as an infrared absorber. From observations at arctic bases and at high altitude flights were missile tests were conducted, he concluded that it was not. But concluding this was one thing, proving it was another.
In 1953 Dr. Plass moved from Canada to southern California to work with Lockheed on missile testing and guidance. And for the first time he had access to a computer. As a competent physicist, Dr. Plass knew how to craft programs to analyze the absorption of infrared radiation by carbon dioxide using quantum mechanics. Without a computer, he would never have been able to make the calculations. Dr. Plass felt confident enough in his belief that our carbon dioxide emissions would warm the Earth's climate that in 1953 he contributed to an article in Time magazine saying so. But the computers of the 1950s were balky and slow, and he had to do his research on his own time. So it took him more than 2 years to mathematically prove that infrared absorption was not saturated at current levels of carbon dioxide in the atmosphere.
Dr. Plass published his work in the July 1956 issue of American Scientist. Dr. Plass made some errors that oddly enough, cancelled each other out. Dr. Plass underestimated the amount of carbon dioxide humankind was emitting into the atmosphere---he gave a figure of 6 billion tons. We now know it was 8.8 billion tons in 1956. Dr. Plass also overestimated the radiative forcing of additional carbon dioxide in the atmosphere. Dr. Plass estimated that a doubling of carbon dioxide in the atmosphere would yield a radiative forcing of 8.3 watts per square meter under clear conditions, and of 5.8 watts per square meter under cloudy conditions. He only had observational from a few arctic bases and brief airborne tests piggybacking on missile testing. The explosion in the earth sciences generated by the 1957-1958 International Geophysical Year, in which high altitude observations were made in the Andes and Antarctica refined this to 4 watts per square meter, under both clear and cloudy conditions. These refinements came quickly---by 1960 all atmospheric physicists knew that a doubling of carbon dioxide would have the correct, 4 watts per square meter warming. And anyone who still goes by the Ångström experiments of 1901-1902 can be dismissed as an ignorant quack (it is amazing how much Ångström's experiments are still cited by deniers).
Dr. Plass's paper is summarized and discussed here.
Dr. Plass made several simplifying assumptions. He assumed no change in water vapor, and no change in absorption of carbon dioxide by the oceans---as I said he was not a climatologist or meteorologist, or oceanographer. He simply ignored feedbacks in his paper. He also assumed that humankind's carbon dioxide emissions into the atmosphere would remain constant at 6 billion tons per year (and as we know, it was already 8.8 billion tons in 1956.
Dr. Plass made some of these simplifying assumptions because of 'known unknowns'--he knew he was not qualified to assume how water vapor and other feedbacks would behave. Also, in the 1950s, while computers were beginning to be used in science, their power was extremely limited by today's standards.
The assumption that carbon dioxide emissions would remain constant seem more inexplicable. As I have discussed in previous blog entries, in the 1950s long-term economic growth on a planet-wide scale was not a given. Countries such as France, Germany, and Japan had lower industrial output in 1950 than in 1913. The United Kingdom and the Soviet Union were not much better. Yes by the mid 1950s the industrial output of the United States was more than triple its 1913 level, and so were Canada's and Australia's. But among those three, only the United States was emitting carbon dioxide at a globally significant level.
This seems amazing, when we consider than global carbon dioxide emissions more than doubled over the next 15 years. But scientists had no way to know that was going to happen--wars had set back economic growth on a generational scale twice in the recent past, and there was no reason to suppose that would not happen again.
Dr. Plass concluded that carbon dioxide could double over a century and raise global temperatures 1.5° C over the next century, a figure that agrees closely with the definitive Charney report of 1979, which gives a 1.2 °C figure. Dr. Plass also concluded that known reserves of carbon-based fossil fuels would add enough CO2 to the atmosphere to warm the surface of the Earth by 7 °C (12.6 °F) by 3000 CE. At such a planetary average surface temperature, the Greenland and West Antarctic ice sheets would be gone, and the East Antarctic ice sheet would be going.
I must repeat here that Dr. Plass was not a climatologist or meteorologist. He did not try to compute feedbacks such as decreasing albedo or increased water vapor in the atmosphere. He focused on the radiative properties of CO2 only.
During the 1960s, returning to his work on CO2 and the Earth's climate, he concluded that net feedbacks were positive, and that each doubling of CO2 in the atmosphere would increase surface temperatures by 3.6 °C.
It has to be said that Dr. Plass did not optimally research and craft his meteorology papers. His lack of some knowledge of meteorology led him to some errors---he tried to compute atmospheric properties and constants that had been solved by others, sometimes decades previously. And he made some mistakes. Today's research on climate feedbacks produce much larger increases in surface temperature.
But I must also repeat that Dr. Plass's proof that increased CO2 in the atmosphere increases infrared radiation absorption did hold. No meteorologist or climatologist denies that now. Not reputable ones.
Roger Revelle had shown how oceanic chemistry buffers prevent the oceans from absorbing all our carbon dioxide emissions. Plass had proven that carbon dioxide was not saturated in the atmosphere from an infrared radiation absorption standpoint. But were human activities really causing carbon dioxide to accumulate in the atmosphere? That question still remained.
And Charles Keeling was to definitively answer it. But that's for the next blog entry.
Here is a picture of Dr. Gilbert Plass:
Dr. Plass left Lockheed in 1960 to join the research staff of Ford's aeronautical division. Dr. Plass also edited Infrared Physics and Technology, a peer-reviewed scientific publication. Dr. Plass worked there until 1963, when he accepted a position as first professor of atmospheric and space sciences with the University of Texas at Arlington, where he remained for 5 years. In 1968 he joined the faculty of Texas A&M University, ultimately becoming head of the department of physics.
Tuesday, May 10, 2011
More entries soon!
Taken a rest from my history of global warming studies blog, been organizing new entries in my head. But more entries are coming soon I promise!
In the meantime a cartoon by the 2010 Pulitzer prize winner for editorial cartoons, Mike Keefe:
And I like this new video, adding it back after the outage:
And...for the very first time on record, the concentration of CO2 exceeded 393 ppm at Mauna Loa, during April 2011.
Which fits well with my new entry about Dr. Charles Keeling, coming soon!
In the meantime a cartoon by the 2010 Pulitzer prize winner for editorial cartoons, Mike Keefe:
And I like this new video, adding it back after the outage:
And...for the very first time on record, the concentration of CO2 exceeded 393 ppm at Mauna Loa, during April 2011.
Which fits well with my new entry about Dr. Charles Keeling, coming soon!
Saturday, April 9, 2011
Roger Revelle' work and what it shows about the carbon budget.
As we have seen from the previous blog entry, the buffering of the oceans works more efficiently the warmer the Earth is. In other words, the warmer the oceans, the quicker carbon dioxide is returned to the atmosphere.
An interesting facet of the ice age cycles is that ice age cycles are just as much about where carbon dioxide is stored as where water and ice are stored. During ice ages, a considerable fraction of the Earth's surface water is stored in ice sheets on the continents, and the oceans fall. The amount of water on the surface of the Earth doesn't change, but where the water is changes.
The same thing happens with carbon dioxide.
During an ice age, the amount of CO2 in the atmosphere falls by about 100 ppm from the 280-300 ppm in the atmosphere during interglacials to 180-200 ppm during the depths of ice ages. But where does the CO2 go?
The answer is that it goes into the sea. The oceans absorb it, and become slightly more acid. There is a cycle that slowly changes the PH of the oceans by ~0.03 The PH of the oceans is not just controlled by the acidic and alkaline compounds dissolved within it, but also by activity factors that mitigate (or increase) ionic concentrations. The chemistry for activity factors in the oceans is very complicated and I will not be going into it here, but the net effect is to slightly mitigate, or lessen, the swings in ionic concentrations in the oceans as the amount of CO2 increases or decreases. At least in the natural history of our recent ice ages. I will discuss it briefly at the end of this entry
Some of the results of this carbon cycle are counterintuitive. During ice ages, the atmosphere has less carbon dioxide, but the oceans are more acid. How can this be?
The answer is that the amount of carbon dioxide in the oceanic/atmospheric system remains broadly the same. At least the carbon does. During ice ages, more carbon remains in methane, which is trapped in methane hydrates in cold continental shelves. The total amount of carbon dioxide in the oceans and atmosphere does fall slightly, with more methane. But the amounts of carbon remain the same.
Another factor is that colder waters can hold more dissolved oxygen, and colder waters can support more life, if other trace minerals needed are present. Evidence does suggest that oceanic biological productivity was slightly greater during ice ages, and this may also have trapped some more gigatons of carbon.
But the main thing is that carbon dioxide accumulated in the oceans.
As I said, the PH of the oceans falling as CO2 concentrations fall in the atmosphere sounds counterintuitive, but it does make sense. The amount of carbon in the oceanic/atmospheric system remains broadly constant. If there is less in the air, there is more in the sea.
Carbon dioxide was not trapped on land in vegetation. Not only were millions of square miles covered under ice sheets, but today's temperate zones were much colder and drier. Forests are the main way life stores carbon on land, and there was much less forest area during the ice ages. There was increased land areas during ice ages as continental shelves were exposed (the area of ice-free land was about the same during ice ages as today) But it was mostly dry tundra or grasslands, and even in the tropics forests shrank and became patchy in restricted areas or river valleys.
*note* Tundra can trap large amounts of carbon. If it is wet tundra. We all know about the thawing tundra bogs bubbling with methane as they thaw. But dry tundra is different. It's just frozen without much biological productivity and not much buried biological matter to become peat infused with methane. The climate of the Earth was much drier during ice ages---so dry that in many places cold enough to form ice sheets, such as Siberia, they did not form. Dust deposits, or loess, show that at best much of North America, Europe, South America and the remaining temperate parts of Asia were at best semi-arid and most of these areas were true deserts.
Much of the interior of North America resembled the Gobi Desert. The Sand Hills of Nebraska were giant sand dunes. When Native Americans first became numerous ~15,000 years ago, the climate was already becoming milder and wetter, supporting more vegetation and life. And I am skipping over the raging question of when mankind arrived in the Americas, but there is not evidence of widespread populations more than 15,000 years ago.
*end note*
As the implications of Revelle's worked seeped through the geologic, oceanographic, biologic, and climatologic branches of science during the 1960s and 1970s, there was some thought that there might be a global carbon cycle, driven perhaps by a long cycle in volcanic activity over tens of thousands of years, that injected and withdrew carbon from the oceanic/atmospheric system. But it was never a widespread belief, and no evidence has been found for it.
The work on the Earth's carbon budget has had two main implications for climate science and global warming, one well grounded in fact, and the other more speculative.
The first one is that yes, warming out of the ice ages does come before carbon dioxide begins to rise in significant quantities. And it does make sense and doesn't invalidate carbon dioxide as the major driver in climate change.
The reason is this. The Milanković cycles determine how much solar energy falls in the polar and temperate zones, and the tropics as well. The Milanković cycles trigger a small warming, which then increases the buffering of CO2 by the oceans. The oceans, as they warm slightly, return CO2 more quickly to the atmosphere. This increases the warming, which then increases the rate CO2 is returned to the atmosphere, and it triggers an accelerating feedback. As the climate warms and becomes wetter, tropical forests and wetlands increase, increasing wetlands and methane emissions. More warming. Methane hydrates in marginal areas become unstable and release their methane. More warming. Swamps and wetlands increase in temperate and polar zones and emit more methane--more warming. The methane is quickly oxidized to CO2 and water, but the CO2 is still a warming gas, as we know.
As ice sheets shrink, the Earth's albedo decreases and the Earth retains more heat. More warming. Less known is the fact that forests are quite dark, while deserts are reflective. Compare the Amazon rain forest to the Sahara Desert in satellite pictures. Or the Siberian taiga to the rocky wastes of northern Canada's islands.
All these effects produce enough warming to bring the Earth out of an ice age. But the key is that temperatures rise first from the Milanković cycle.
This is a point that deniers try to exploit. When they do so, you can be sure that they are either ignorant of climate processes or being deliberately dishonest. Usually they are being dishonest.
Deniers argue that because the temperature began to rise before CO2 began to rise in the atmosphere that means that CO2 is not a greenhouse gas. Or that it doesn't trigger warming. Or some such thing. No. CO2 rises as a feedback to a slight temperature increase, and then vastly increases the temperature rise far beyond what Milanković cycles can do. And the albedo effects from reductions in ice and snow cover and changes in vegetation increase temperatures still further! The Milanković cycle increases temperatures a few tenths of a degree and the feedbacks from CO2 and decreasing albedo trigger the far greater temperature rises.
Milanković cycles work because the Earth is finely balanced between different climatic states, ice ages and interglacials. Milanković cycles determine how much solar radiation reaches the polar and adjacent temperate zones. Aside from changes in the eccentricity of the orbit of the Earth, Milanković cycles do not change the total amount of solar radiation reaching the earth. When changes in the axial tilt increase solar radiation in polar zones, they decrease solar radiation in tropical zones. The total amount of solar radiation reaching the Earth remains the same. Aside from the changes in the eccentricity of the orbit of the Earth around the sun, Milanković cycles wouldn't change the temperature of the Earth at all if positive feedbacks didn't come into play when more solar radiation reaches polar and adjacent temperate zones.
The fact that the Earth's climate warms so much from subtle changes in solar radiation in polar zones shows that the positive feedbacks are very strong. And that is why what we are doing to the atmosphere is so dangerous.
This brings into play climate sensitivity. We know that Milanković cycles, aside from the minor orbital eccentricity effect, can't change the total solar radiation the Earth receives. Jule Gregory Charney (1917-1981) crafted the first definitive report on climate sensitivity in 1979. You can read it here.
Climate sensitivity compares how much a given increase in a greenhouse gas, carbon dioxide in this care, to what actually happened in the climatic record. Although Charney's report is from 1979, it is definitive. The basic physics of radiation absorption by CO2 have been well understood for decades (As I have said in previous entries, it was believed until the 1940s that CO2 in the atmosphere was saturated as far as infrared radiation absorption is concerned. In other words, that adding more CO2 would not make a difference because it already absorbed all the infrared radiation it could. I will be discussing how that was proved wrong soon in an upcoming blog entry.)
From the Charney report we know that climate sensitivity greatly increases temperature swings from changes in atmospheric carbon dioxide alone would do. And has done. The question humanity faces is how powerful these positive feedbacks will be in a warming world.
Revelle's research, and other research by scientists later, is disquieting on several fronts. Back then there was 50 times as much dissolved CO2 in the oceans as in the atmosphere. It is now about 40 times as much, as atmospheric CO2 has increased so quickly. We have added nearly 3 trillion tons of carbon dioxide to the atmospheric/oceanic system. Despite the swings in oceanic chemistry between ice ages and interglacials, the oceans are already far more acid (or less alkaline) than in the ice ages. As temperatures rise, the efficiency of carbon dioxide return to the atmosphere increases. The oceans hold hundreds of trillions of tons of carbon dioxide. Could the large increases in temperature in store turn the oceans into net carbon dioxide emitters?
We don't know.
The warmer water becomes, the less gas it can dissolve. That runs counter to what we know in daily life, because we know about how solids behave in water. Cold water doesn't dissolve much sugar, and dissolves it much more slowly than hot water.
With gases it is different. Molecules vibrate faster and travel faster as temperatures warm. In fact the motion of molecules defines temperature in our daily lives. Solids dissolve more readily in liquids as the temperature rises because the break off from the surface of solids and are incorporated into the liquid.
For gases, the greater the temperature, the more rapidly the gas molecules travel, and the more easily they can escape the liquid. That is why the warmer water is, the less dissolved gas they can hold. That is why CO2 buffering becomes more efficient as temperatures rise. Rising temperatures will also decrease the amount of oxygen dissolved in water, with impacts on biological productivity. We don't know how oceanic life will adapt to warmer, more acidic conditions. Photosynthetic algae do remove a lot of CO2 and convert it to oxygen. Algae decompose on the surface when they die, and the carbon within them remains part of the open carbon system. But animals feeding on them can sink to the ocean floor when they die, as well as diatom shells. Could the warming and acidification of the oceans decrease biological productivity enough to significantly reduce the amount of carbon that settles to the sea floors?
We don't know.
The chemical processes of the Revelle Effect are well known, and assuming no major changes in biological activity, the impact of the Revelle effect is quantifiable. All measurements agree that CO2 buffering and return to the atmosphere increases by 6%-8% for every 1° C. That is a closer agreement than many in science.
But the possibility of some sort of biological threshold being reached--a cliff--where biological productivity decreases enough to increase the Revelle effect much more than expected is not something we can safely ignore.
Here are some links to the chemistry of oceanic carbon dioxide buffering:
From the IPCC
Some of the major chemical reactions in oceanic carbon dioxide buffering from Columbia University.
And a more detailed paper on the chemical reactions of oceanic carbon dioxide buffering by Chuixiang Yi, Peng Gong, Ming Xu and Ye Qi.
An interesting facet of the ice age cycles is that ice age cycles are just as much about where carbon dioxide is stored as where water and ice are stored. During ice ages, a considerable fraction of the Earth's surface water is stored in ice sheets on the continents, and the oceans fall. The amount of water on the surface of the Earth doesn't change, but where the water is changes.
The same thing happens with carbon dioxide.
During an ice age, the amount of CO2 in the atmosphere falls by about 100 ppm from the 280-300 ppm in the atmosphere during interglacials to 180-200 ppm during the depths of ice ages. But where does the CO2 go?
The answer is that it goes into the sea. The oceans absorb it, and become slightly more acid. There is a cycle that slowly changes the PH of the oceans by ~0.03 The PH of the oceans is not just controlled by the acidic and alkaline compounds dissolved within it, but also by activity factors that mitigate (or increase) ionic concentrations. The chemistry for activity factors in the oceans is very complicated and I will not be going into it here, but the net effect is to slightly mitigate, or lessen, the swings in ionic concentrations in the oceans as the amount of CO2 increases or decreases. At least in the natural history of our recent ice ages. I will discuss it briefly at the end of this entry
Some of the results of this carbon cycle are counterintuitive. During ice ages, the atmosphere has less carbon dioxide, but the oceans are more acid. How can this be?
The answer is that the amount of carbon dioxide in the oceanic/atmospheric system remains broadly the same. At least the carbon does. During ice ages, more carbon remains in methane, which is trapped in methane hydrates in cold continental shelves. The total amount of carbon dioxide in the oceans and atmosphere does fall slightly, with more methane. But the amounts of carbon remain the same.
Another factor is that colder waters can hold more dissolved oxygen, and colder waters can support more life, if other trace minerals needed are present. Evidence does suggest that oceanic biological productivity was slightly greater during ice ages, and this may also have trapped some more gigatons of carbon.
But the main thing is that carbon dioxide accumulated in the oceans.
As I said, the PH of the oceans falling as CO2 concentrations fall in the atmosphere sounds counterintuitive, but it does make sense. The amount of carbon in the oceanic/atmospheric system remains broadly constant. If there is less in the air, there is more in the sea.
Carbon dioxide was not trapped on land in vegetation. Not only were millions of square miles covered under ice sheets, but today's temperate zones were much colder and drier. Forests are the main way life stores carbon on land, and there was much less forest area during the ice ages. There was increased land areas during ice ages as continental shelves were exposed (the area of ice-free land was about the same during ice ages as today) But it was mostly dry tundra or grasslands, and even in the tropics forests shrank and became patchy in restricted areas or river valleys.
*note* Tundra can trap large amounts of carbon. If it is wet tundra. We all know about the thawing tundra bogs bubbling with methane as they thaw. But dry tundra is different. It's just frozen without much biological productivity and not much buried biological matter to become peat infused with methane. The climate of the Earth was much drier during ice ages---so dry that in many places cold enough to form ice sheets, such as Siberia, they did not form. Dust deposits, or loess, show that at best much of North America, Europe, South America and the remaining temperate parts of Asia were at best semi-arid and most of these areas were true deserts.
Much of the interior of North America resembled the Gobi Desert. The Sand Hills of Nebraska were giant sand dunes. When Native Americans first became numerous ~15,000 years ago, the climate was already becoming milder and wetter, supporting more vegetation and life. And I am skipping over the raging question of when mankind arrived in the Americas, but there is not evidence of widespread populations more than 15,000 years ago.
*end note*
As the implications of Revelle's worked seeped through the geologic, oceanographic, biologic, and climatologic branches of science during the 1960s and 1970s, there was some thought that there might be a global carbon cycle, driven perhaps by a long cycle in volcanic activity over tens of thousands of years, that injected and withdrew carbon from the oceanic/atmospheric system. But it was never a widespread belief, and no evidence has been found for it.
The work on the Earth's carbon budget has had two main implications for climate science and global warming, one well grounded in fact, and the other more speculative.
The first one is that yes, warming out of the ice ages does come before carbon dioxide begins to rise in significant quantities. And it does make sense and doesn't invalidate carbon dioxide as the major driver in climate change.
The reason is this. The Milanković cycles determine how much solar energy falls in the polar and temperate zones, and the tropics as well. The Milanković cycles trigger a small warming, which then increases the buffering of CO2 by the oceans. The oceans, as they warm slightly, return CO2 more quickly to the atmosphere. This increases the warming, which then increases the rate CO2 is returned to the atmosphere, and it triggers an accelerating feedback. As the climate warms and becomes wetter, tropical forests and wetlands increase, increasing wetlands and methane emissions. More warming. Methane hydrates in marginal areas become unstable and release their methane. More warming. Swamps and wetlands increase in temperate and polar zones and emit more methane--more warming. The methane is quickly oxidized to CO2 and water, but the CO2 is still a warming gas, as we know.
As ice sheets shrink, the Earth's albedo decreases and the Earth retains more heat. More warming. Less known is the fact that forests are quite dark, while deserts are reflective. Compare the Amazon rain forest to the Sahara Desert in satellite pictures. Or the Siberian taiga to the rocky wastes of northern Canada's islands.
All these effects produce enough warming to bring the Earth out of an ice age. But the key is that temperatures rise first from the Milanković cycle.
This is a point that deniers try to exploit. When they do so, you can be sure that they are either ignorant of climate processes or being deliberately dishonest. Usually they are being dishonest.
Deniers argue that because the temperature began to rise before CO2 began to rise in the atmosphere that means that CO2 is not a greenhouse gas. Or that it doesn't trigger warming. Or some such thing. No. CO2 rises as a feedback to a slight temperature increase, and then vastly increases the temperature rise far beyond what Milanković cycles can do. And the albedo effects from reductions in ice and snow cover and changes in vegetation increase temperatures still further! The Milanković cycle increases temperatures a few tenths of a degree and the feedbacks from CO2 and decreasing albedo trigger the far greater temperature rises.
Milanković cycles work because the Earth is finely balanced between different climatic states, ice ages and interglacials. Milanković cycles determine how much solar radiation reaches the polar and adjacent temperate zones. Aside from changes in the eccentricity of the orbit of the Earth, Milanković cycles do not change the total amount of solar radiation reaching the earth. When changes in the axial tilt increase solar radiation in polar zones, they decrease solar radiation in tropical zones. The total amount of solar radiation reaching the Earth remains the same. Aside from the changes in the eccentricity of the orbit of the Earth around the sun, Milanković cycles wouldn't change the temperature of the Earth at all if positive feedbacks didn't come into play when more solar radiation reaches polar and adjacent temperate zones.
The fact that the Earth's climate warms so much from subtle changes in solar radiation in polar zones shows that the positive feedbacks are very strong. And that is why what we are doing to the atmosphere is so dangerous.
This brings into play climate sensitivity. We know that Milanković cycles, aside from the minor orbital eccentricity effect, can't change the total solar radiation the Earth receives. Jule Gregory Charney (1917-1981) crafted the first definitive report on climate sensitivity in 1979. You can read it here.
Climate sensitivity compares how much a given increase in a greenhouse gas, carbon dioxide in this care, to what actually happened in the climatic record. Although Charney's report is from 1979, it is definitive. The basic physics of radiation absorption by CO2 have been well understood for decades (As I have said in previous entries, it was believed until the 1940s that CO2 in the atmosphere was saturated as far as infrared radiation absorption is concerned. In other words, that adding more CO2 would not make a difference because it already absorbed all the infrared radiation it could. I will be discussing how that was proved wrong soon in an upcoming blog entry.)
From the Charney report we know that climate sensitivity greatly increases temperature swings from changes in atmospheric carbon dioxide alone would do. And has done. The question humanity faces is how powerful these positive feedbacks will be in a warming world.
Revelle's research, and other research by scientists later, is disquieting on several fronts. Back then there was 50 times as much dissolved CO2 in the oceans as in the atmosphere. It is now about 40 times as much, as atmospheric CO2 has increased so quickly. We have added nearly 3 trillion tons of carbon dioxide to the atmospheric/oceanic system. Despite the swings in oceanic chemistry between ice ages and interglacials, the oceans are already far more acid (or less alkaline) than in the ice ages. As temperatures rise, the efficiency of carbon dioxide return to the atmosphere increases. The oceans hold hundreds of trillions of tons of carbon dioxide. Could the large increases in temperature in store turn the oceans into net carbon dioxide emitters?
We don't know.
The warmer water becomes, the less gas it can dissolve. That runs counter to what we know in daily life, because we know about how solids behave in water. Cold water doesn't dissolve much sugar, and dissolves it much more slowly than hot water.
With gases it is different. Molecules vibrate faster and travel faster as temperatures warm. In fact the motion of molecules defines temperature in our daily lives. Solids dissolve more readily in liquids as the temperature rises because the break off from the surface of solids and are incorporated into the liquid.
For gases, the greater the temperature, the more rapidly the gas molecules travel, and the more easily they can escape the liquid. That is why the warmer water is, the less dissolved gas they can hold. That is why CO2 buffering becomes more efficient as temperatures rise. Rising temperatures will also decrease the amount of oxygen dissolved in water, with impacts on biological productivity. We don't know how oceanic life will adapt to warmer, more acidic conditions. Photosynthetic algae do remove a lot of CO2 and convert it to oxygen. Algae decompose on the surface when they die, and the carbon within them remains part of the open carbon system. But animals feeding on them can sink to the ocean floor when they die, as well as diatom shells. Could the warming and acidification of the oceans decrease biological productivity enough to significantly reduce the amount of carbon that settles to the sea floors?
We don't know.
The chemical processes of the Revelle Effect are well known, and assuming no major changes in biological activity, the impact of the Revelle effect is quantifiable. All measurements agree that CO2 buffering and return to the atmosphere increases by 6%-8% for every 1° C. That is a closer agreement than many in science.
But the possibility of some sort of biological threshold being reached--a cliff--where biological productivity decreases enough to increase the Revelle effect much more than expected is not something we can safely ignore.
Here are some links to the chemistry of oceanic carbon dioxide buffering:
From the IPCC
Some of the major chemical reactions in oceanic carbon dioxide buffering from Columbia University.
And a more detailed paper on the chemical reactions of oceanic carbon dioxide buffering by Chuixiang Yi, Peng Gong, Ming Xu and Ye Qi.
Sunday, April 3, 2011
Roger Revelle
The investigation into atmospheric CO2 and its interaction with the oceans is a long and complex story. Dozens of oceanographers and chemists contributed in this work, and the chemistry is also very complex. However, Roger Revelle is the central figure in this field of research. At least I consider him so. At any rate, he was the first to show that mankind's addition of CO2 to the atmosphere would not be absorbed by the oceans quickly, and was able to work out why.
This had been a question since Arrhenius. It was known since the Challenger Expedition of the 1870s that the oceans contain large amounts of CO2, and that oceans are alkaline world-wide. The Challenger expedition showed that the oceans contained ~50 times as much CO2 as the atmosphere. One of the main objections to anthropogenic global warming is that the oceans are alkaline---and should therefore absorb CO2 easily.
But proponents of anthropogenic global warming, such as Arrhenius, Alfred Wallace, and Callendar raised an interesting question. If the oceans really could absorb all the anthropogenic emissions of CO2 easily, why didn't the oceans absorb all the CO2 that is in the air now? In other words, since the oceans hold 50 times as much CO2 as the atmosphere, why didn't the oceans just absorb the 51st molecule, and then have the Earth freeze into a snowball?
This was a nagging question for oceanographers, but that scientific field was consumed by another controversy. As I wrote in a previous blog entry, the oceans have contained roughly the same salt concentration as today for billions of years. Once it was realized that the Earth was billions of years old, the main question for oceanographers was how do the oceans get rid of their salt? Even now some aspects of that question have not been solved, although we now have a broad picture of how salt can be evaporated and buried under sediments in shallow seas and estuaries. But during much of the 20th century, the salt question was the major question in oceanography.
Roger Revelle (1909-1991) was an oceanographer with the Scripps Institute of Oceanography. During the mid 1950s he was part of a team studying how fast the oceans 'turn over', the seawater at the surface sinking to the depths and deep ocean waters rising to the surface. This was suddenly an important question. The Japanese were in an uproar over nuclear testing and radioactive pollution of their Pacific fishing grounds.
There had been rising anxiety in Japan already about nuclear fallout (Japan had great nuclear anxiety in any case from their experience with the atomic bombing of Hiroshima and Nagasaki less than 10 years earlier.) In 1954 two incidents occurred.
The first, and most serious, was the irradiation of the Daigo Fukuryū Maru (q.v) and later that year, the release of the movie Gojira, which we know as Godzilla, rushed into production after the Daigo Fukuryū Maru incident.
The United States Navy rushed a study to find out how fast the oceans 'turned over' and carried radioactive fallout to the depths. Revelle and his team determined that the oceans turned over over several hundred years (that is a bit wrong---we no know that the oceans turn over in about 3,000 years). That data showed the oceans turn over fast enough to absorb and remove CO2 from anthropogenic emissions. And yes, 3,000 years is fast enough also to dissolve most CO2 in the oceans and keep atmospheric CO2 from rising much.
But Revelle went further. The question of why the oceans didn't absorb all CO2 nagged at him. And his research in the field gave him knowledge and access to a tool oceanographers didn't have. The nuclear tests in the Pacific created lots of radioactive carbon isotopes. Carbon isotopes as great as C-22 and as low as C-8 were created. Most of these had a half life of microseconds or less. But C-11 (carbon 11) has a half-life a little over 20 minutes. Revelle didn't use the C-11 created by nuclear explosions---almost all would be gone in a couple days--too quick to visit an explosion site, with lots of other longer-lived radioisotopes around. But C-11 did give him an idea---create CO2 using C-11 and see how it interacted with ocean water. It was radioactive enough to be very easily traceable, but not too fast to decay immediately. And also, in a day or two almost all the C-11 would be gone. So it wasn't a disposal hazard.
The chemistry he found was amazingly complicated. Seawater is not just salt, it is a complex soup of many thousands of chemicals dissolved within it. And it also has living organisms. So there are thousands of reactions that CO2 can make with the different chemicals in seawater.
It had been suspected that the oceans had a buffering mechanism. What Revelle found was that in many cases, CO2 combined with chemicals in the seawater and created volatile compounds that promptly evaporated back into the air. Once back in the atmosphere, the CO2 would encounter free oxygen, or be dissociated by ultraviolet light, and create CO2. When he raised CO2 concentrations slightly, to 350 ppm or 400 ppm in the atmospheric samples over the tanks of seawater, molecules containing the radioactive C-11 were returned back to the air in significant amounts, while significantly less C-11 remained in the seawater. C-11 decays too quickly for longer studies, so Revelle switched to C-14, with a half-life of 5,730 years. In 1955-56 he determined that when CO2 in the atmosphere increased, about half of what the oceans absorbed would be evaporated out via volatile organic compounds within a year.
In a paper he co-authored with Dr. Hans Seuss (1909-1993) (no, not that Dr. Seuss) Revelle wrote in a few sentences at the end that assuming that CO2 emissions stayed at 1957 levels, CO2 would rise in the atmosphere about 40% (to 440 ppm) over the next few centuries and stabilize.
This was mind-blowing. 440 ppm was a big rise! And certainly enough to warm the Earth's climate considerably! Revelle was not a climatologist or meteorologist, and did not realized the implications of what he had written. But others did.
It created a big scientific controversy. Unlike today, it played out in scientific circles and was largely unreported to the public. Objections were made, and then refuted. Perhaps the C-11 (with a half-life of 20 minutes, it is very radioactive) was killing the seawater microbes and they were releasing volatile organic compounds when they died. But research by others using C-14 quickly showed this was not the case. By 1960 Revelle's work was accepted.
One of the scientists Revelle worked with was Dr. Charles Keeling. Keeling, whom we all know, was inspired by Revelle to create his famous Mauna Loa carbon dioxide measuring observatory. It also combined with other work that I will be talking about in another blog entry, about how CO2's apparent saturation in its IR bands was not really saturated after all to show that increasing CO2 in the atmosphere would result in global warming. This has been the consensus for the past 50 years, and is the consensus now.
Revelle's guess about future CO2 concentrations in the atmosphere was a gross underestimate. He assumed that CO2 emissions would remain close to their 1957 levels. At the time, that was not a ridiculous assumption.
During the previous generation, there had been two terrible world wars. Continuous, progressive, exponential economic growth had not been the reality. In 1950, industrial production in the Soviet Union, Germany, France, Italy and Japan was lower than it was in 1913! There was no real reason to expect that the future would be different from the past. What Revelle didn't realize was that beginning in the mid 1950s the world had entered an unprecedented economic boom---with CO2 emissions more than doubling between 1957 and 1972. From 1973 to the late 1990s, emissions slowed in their rate of increase, but did not stop rising. And the growing boom in China and India has caused CO2 emissions to rise more rapidly in the past 15 years than during the pause from the mid 70s to the mid 90s.
In short CO2 emissions rose on an annual basis
1957-1973 6%
1974-1997 2%
1998-2011 3.5% (4%+ 2005-2010, despite the recent recession)
The combination of unprecedented economic growth, Revelle's CO2 chemistry work, Keeling's Curve, the discovery that IR absorption by CO2 was not saturated led by 1965 to a scientific conference in Boulder, CO about anthropogenic global warming--the first scientific conference with that as the main topic. I'll blog about that soon.
Revelle's work was also incomplete. He identified some of the main chemical pathways CO2 dissolved in the oceans returns to the atmosphere in volatile compounds. But many other oceanographers and chemists have been working since to identify other pathways--there are tens of thousands of them! Individually, most are trivial, but together they make a significant fraction of carbon dioxide atmospheric return. They vary by differences in temperature, local differences in chemicals dissolved in the oceans, and of course, different organisms present in the surface waters.
Also, some of the major chemical reactions that return CO2 to the atmosphere had already been discovered during the 1940s and 1930s. But it wasn't realized that they were a major part of the flux of carbon between the atmosphere and oceans. What Revelle largely did was discover that these reactions returned a large amount of carbon to the atmosphere quickly. There is a distinction between discovering a chemical reaction and discovering its magnitude and importance.
One alarming thing is that this return of carbon dioxide to the atmosphere is a powerful feedback. The warmer surface temperatures rise, the more quickly organic compounds evaporate and return to the atmosphere. Also, as temperatures rise, more compounds become able to evaporate.
Research shows, and is unanimous in agreement, that for each 1 C° rise in the surface ocean temperature, the return rate of CO2 to the atmosphere increases by 7%.
When I said unanimous, I was not quite right. All models show an increase from 6-8% per degree Celsius. What's really interesting is that if the increase is closer to 8% then more CO2 will be returned to the atmosphere, warming it faster, and cause more CO2 to be emitted by the oceans. This has big implications on temperature and oceanic acidification. It results in a damned either way situation. A slightly faster CO2 evaporation rate will result in faster warming, but less oceanic acidification. A slower CO2 evaporation rate will result in less warming, but more acidification. A difference between 6.2% and 7.8% is pretty close agreement, but running the calculations out to 2100 and beyond results in quite different states for the atmosphere and the oceans. As always, we need more research!
Roger Revelle
I will add further information in this blog entry later on some of the chemical pathways of CO2 return to the atmosphere.
This had been a question since Arrhenius. It was known since the Challenger Expedition of the 1870s that the oceans contain large amounts of CO2, and that oceans are alkaline world-wide. The Challenger expedition showed that the oceans contained ~50 times as much CO2 as the atmosphere. One of the main objections to anthropogenic global warming is that the oceans are alkaline---and should therefore absorb CO2 easily.
But proponents of anthropogenic global warming, such as Arrhenius, Alfred Wallace, and Callendar raised an interesting question. If the oceans really could absorb all the anthropogenic emissions of CO2 easily, why didn't the oceans absorb all the CO2 that is in the air now? In other words, since the oceans hold 50 times as much CO2 as the atmosphere, why didn't the oceans just absorb the 51st molecule, and then have the Earth freeze into a snowball?
This was a nagging question for oceanographers, but that scientific field was consumed by another controversy. As I wrote in a previous blog entry, the oceans have contained roughly the same salt concentration as today for billions of years. Once it was realized that the Earth was billions of years old, the main question for oceanographers was how do the oceans get rid of their salt? Even now some aspects of that question have not been solved, although we now have a broad picture of how salt can be evaporated and buried under sediments in shallow seas and estuaries. But during much of the 20th century, the salt question was the major question in oceanography.
Roger Revelle (1909-1991) was an oceanographer with the Scripps Institute of Oceanography. During the mid 1950s he was part of a team studying how fast the oceans 'turn over', the seawater at the surface sinking to the depths and deep ocean waters rising to the surface. This was suddenly an important question. The Japanese were in an uproar over nuclear testing and radioactive pollution of their Pacific fishing grounds.
There had been rising anxiety in Japan already about nuclear fallout (Japan had great nuclear anxiety in any case from their experience with the atomic bombing of Hiroshima and Nagasaki less than 10 years earlier.) In 1954 two incidents occurred.
The first, and most serious, was the irradiation of the Daigo Fukuryū Maru (q.v) and later that year, the release of the movie Gojira, which we know as Godzilla, rushed into production after the Daigo Fukuryū Maru incident.
The United States Navy rushed a study to find out how fast the oceans 'turned over' and carried radioactive fallout to the depths. Revelle and his team determined that the oceans turned over over several hundred years (that is a bit wrong---we no know that the oceans turn over in about 3,000 years). That data showed the oceans turn over fast enough to absorb and remove CO2 from anthropogenic emissions. And yes, 3,000 years is fast enough also to dissolve most CO2 in the oceans and keep atmospheric CO2 from rising much.
But Revelle went further. The question of why the oceans didn't absorb all CO2 nagged at him. And his research in the field gave him knowledge and access to a tool oceanographers didn't have. The nuclear tests in the Pacific created lots of radioactive carbon isotopes. Carbon isotopes as great as C-22 and as low as C-8 were created. Most of these had a half life of microseconds or less. But C-11 (carbon 11) has a half-life a little over 20 minutes. Revelle didn't use the C-11 created by nuclear explosions---almost all would be gone in a couple days--too quick to visit an explosion site, with lots of other longer-lived radioisotopes around. But C-11 did give him an idea---create CO2 using C-11 and see how it interacted with ocean water. It was radioactive enough to be very easily traceable, but not too fast to decay immediately. And also, in a day or two almost all the C-11 would be gone. So it wasn't a disposal hazard.
The chemistry he found was amazingly complicated. Seawater is not just salt, it is a complex soup of many thousands of chemicals dissolved within it. And it also has living organisms. So there are thousands of reactions that CO2 can make with the different chemicals in seawater.
It had been suspected that the oceans had a buffering mechanism. What Revelle found was that in many cases, CO2 combined with chemicals in the seawater and created volatile compounds that promptly evaporated back into the air. Once back in the atmosphere, the CO2 would encounter free oxygen, or be dissociated by ultraviolet light, and create CO2. When he raised CO2 concentrations slightly, to 350 ppm or 400 ppm in the atmospheric samples over the tanks of seawater, molecules containing the radioactive C-11 were returned back to the air in significant amounts, while significantly less C-11 remained in the seawater. C-11 decays too quickly for longer studies, so Revelle switched to C-14, with a half-life of 5,730 years. In 1955-56 he determined that when CO2 in the atmosphere increased, about half of what the oceans absorbed would be evaporated out via volatile organic compounds within a year.
In a paper he co-authored with Dr. Hans Seuss (1909-1993) (no, not that Dr. Seuss) Revelle wrote in a few sentences at the end that assuming that CO2 emissions stayed at 1957 levels, CO2 would rise in the atmosphere about 40% (to 440 ppm) over the next few centuries and stabilize.
This was mind-blowing. 440 ppm was a big rise! And certainly enough to warm the Earth's climate considerably! Revelle was not a climatologist or meteorologist, and did not realized the implications of what he had written. But others did.
It created a big scientific controversy. Unlike today, it played out in scientific circles and was largely unreported to the public. Objections were made, and then refuted. Perhaps the C-11 (with a half-life of 20 minutes, it is very radioactive) was killing the seawater microbes and they were releasing volatile organic compounds when they died. But research by others using C-14 quickly showed this was not the case. By 1960 Revelle's work was accepted.
One of the scientists Revelle worked with was Dr. Charles Keeling. Keeling, whom we all know, was inspired by Revelle to create his famous Mauna Loa carbon dioxide measuring observatory. It also combined with other work that I will be talking about in another blog entry, about how CO2's apparent saturation in its IR bands was not really saturated after all to show that increasing CO2 in the atmosphere would result in global warming. This has been the consensus for the past 50 years, and is the consensus now.
Revelle's guess about future CO2 concentrations in the atmosphere was a gross underestimate. He assumed that CO2 emissions would remain close to their 1957 levels. At the time, that was not a ridiculous assumption.
During the previous generation, there had been two terrible world wars. Continuous, progressive, exponential economic growth had not been the reality. In 1950, industrial production in the Soviet Union, Germany, France, Italy and Japan was lower than it was in 1913! There was no real reason to expect that the future would be different from the past. What Revelle didn't realize was that beginning in the mid 1950s the world had entered an unprecedented economic boom---with CO2 emissions more than doubling between 1957 and 1972. From 1973 to the late 1990s, emissions slowed in their rate of increase, but did not stop rising. And the growing boom in China and India has caused CO2 emissions to rise more rapidly in the past 15 years than during the pause from the mid 70s to the mid 90s.
In short CO2 emissions rose on an annual basis
1957-1973 6%
1974-1997 2%
1998-2011 3.5% (4%+ 2005-2010, despite the recent recession)
The combination of unprecedented economic growth, Revelle's CO2 chemistry work, Keeling's Curve, the discovery that IR absorption by CO2 was not saturated led by 1965 to a scientific conference in Boulder, CO about anthropogenic global warming--the first scientific conference with that as the main topic. I'll blog about that soon.
Revelle's work was also incomplete. He identified some of the main chemical pathways CO2 dissolved in the oceans returns to the atmosphere in volatile compounds. But many other oceanographers and chemists have been working since to identify other pathways--there are tens of thousands of them! Individually, most are trivial, but together they make a significant fraction of carbon dioxide atmospheric return. They vary by differences in temperature, local differences in chemicals dissolved in the oceans, and of course, different organisms present in the surface waters.
Also, some of the major chemical reactions that return CO2 to the atmosphere had already been discovered during the 1940s and 1930s. But it wasn't realized that they were a major part of the flux of carbon between the atmosphere and oceans. What Revelle largely did was discover that these reactions returned a large amount of carbon to the atmosphere quickly. There is a distinction between discovering a chemical reaction and discovering its magnitude and importance.
One alarming thing is that this return of carbon dioxide to the atmosphere is a powerful feedback. The warmer surface temperatures rise, the more quickly organic compounds evaporate and return to the atmosphere. Also, as temperatures rise, more compounds become able to evaporate.
Research shows, and is unanimous in agreement, that for each 1 C° rise in the surface ocean temperature, the return rate of CO2 to the atmosphere increases by 7%.
When I said unanimous, I was not quite right. All models show an increase from 6-8% per degree Celsius. What's really interesting is that if the increase is closer to 8% then more CO2 will be returned to the atmosphere, warming it faster, and cause more CO2 to be emitted by the oceans. This has big implications on temperature and oceanic acidification. It results in a damned either way situation. A slightly faster CO2 evaporation rate will result in faster warming, but less oceanic acidification. A slower CO2 evaporation rate will result in less warming, but more acidification. A difference between 6.2% and 7.8% is pretty close agreement, but running the calculations out to 2100 and beyond results in quite different states for the atmosphere and the oceans. As always, we need more research!
Roger Revelle
I will add further information in this blog entry later on some of the chemical pathways of CO2 return to the atmosphere.
Sunday, March 27, 2011
Harold Urey and Cesare Emiliani
The pause in global warming research continued a surprisingly long time. World War II accounts for part of this--scientists were working on problems directly related to the war effort and not more esoteric research. Afterward, there was the Cold War, in which although funding for scientific research expanded enormously, much continued to be directed towards military applications. This was the case until the International Geophysical Year of 1957-1958, which stimulated much research relevant to anthropogenic global warming, triggering investigations some of which continue to this day. But that's for another entry.
However, not all was quiet on the anthropogenic global warming front. Harold Urey (1893-1981) is one of the towering giants of 20th century science. He won the Nobel Prize for chemistry in 1934 for research in isotopes. He discovered deuterium in 1931, and was the first to isolate pure liquid deuterium from liquid hydrogen. He did much research on the isotopes of uranium, being a member of the brain trust that helped develop the atom (fission) bomb. He came up with a model for the early atmosphere of the Earth in 1952, speculating it was composed of ammonia, methane, and hydrogen, which he published in his book The Planets: Their Origin and Development. Urey's hypothesis for the composition of the atmosphere of the early Earth has since been shown to be wrong, but it was a good kind of wrong that stimulated a lot of research. Harold Urey was Stanley Miller's professor and adviser, and together they crafted the Miller-Urey experiment, one of the most famous experiments of 20th century science. This experiment showed that complex organic compounds, including many amino acids, could be generated easily and in large quantities by natural processes.
Harold Urey was also Isaac Asimov's chemistry professor at Columbia University.
After World War II, Harold Urey turned his attention to isotopes of oxygen. It was a natural question for him to explore. Urey had used centrifuges to separate deuterium from ordinary hydrogen, and had helped work out how to separate uranium-235 from uranium-238 by creating uranium hexafluoride which could be spun in centrifuges to separate the lighter uranium-235 from the heavier uranium-238 (and helped created the more exciting and dangerous world of today)
Harold Urey realized that evaporation of water and condensing it into glaciers could act as a natural way to separate isotopes. Oxygen-18 is a rare but stable isotope of oxygen. A water molecule containing an atom of oxygen-18 is heavier than water molecules containing oxygen-16, and does not evaporate as easily. This means that during ice ages, when more and more evaporated water is trapped in ice sheets, the remaining water in the oceans is enriched in oxygen-18. Therefore, the more oxygen-18 is concentrated in the oceans, the greater the volume of ice sheets and (presumably) the colder the Earth was!
Urey wrote in 1947 that we should check coring samples of the ocean for deposits of foraminifera (forams) shells in the sediments, hypothesizing that those living in times of past ice ages would have enriched levels of oxygen-18 in their shells. This was the first time that nuclear science and biology had been combined to solve a scientific problem!
The problem was taken up by Cesare Emiliani (1922-1995), a geologist from Italy who was one of Urey's students after the war (Many of Urey's students became scientific giants of their own working on problems suggested by Urey).
There were many difficult problems. Sediment coring up until that time was not very sophisticated---it was simply dropping a very heavy and dense metal tube into oceanic sediments. This had been done since the 1870s Challenger expedition, discussed in a previous blog entry, but it blurred and mixed the core samples too much to provide reliable samples of foram shells inside. This is not to say that these primitive core samples were useless---they did provide much information on sediment layers but they were too crude for the sort of research Emiliani was doing.
Borge Kullenberg saved the day. Working on the Swedish Deep Sea Expedition of 1947, he developed a new coring device that used a piston which deployed when the coring tube hit the ocean floor, enabling core sample tubes to be wider and penetrate far deeper into the ocean floor sediments. The coring samples were much clearer, and were 15 meters long, instead of a couple meters. By 1951 he developed a 20 meter coring apparatus.
Back in the lab, people could take precise samples of each layer, tease out a few hundred foram shells, which were then ground and roasted in the presence of pure oxygen-16 gas to form carbon dioxide. The oxygen-18 could then be measured by spinning the carbon dioxide in centrifuges, separating the heavy molecules containing oxygen-18 out.
As Kullenberg's new coring apparatus replaced older coring techniques in a few years, lots of samples became available for inspection. Emiliani used the new technique of carbon dating on the top layers to determine an average rate of sediment deposition on the ocean floor. (beyond about 40,000 years carbon dating does not work as carbon-14 decays). With the new sediment cores of 20 meters, he was able to get samples as much as 300,000 years old.
Emiliani found several pieces of the climate puzzle.
He found that the signature of ice ages could be clearly and consistently seen from ocean-floor sediment samples around the globe.
He also found that the temperature curves generated from these samples matched the Milanković theory very well.
Milanković had not achieved much scientific recognition up until this point---his chronology for ice ages differed from the scientific consensus developed in the late 19th century. But he lived long enough to see vindication through Emiliani's work.
Emiliani also found that there were sharp changes in the temperature of the earth---lots of evidence that ice ages were not smooth curves of cooler and warmer temperatures, but lots of sharp jagged swings in temperature, in periods of hundreds of years. Sharp advances and retreats.
When Emiliani published his research in 1955, it was recognized immediately as groundbreaking. The conclusion that ice ages were driven by Milanković cycles was accepted.
But the sharp temperature swings were not. The coring technique was new--perhaps it was affecting the sampling. The idea that the climate could change by large magnitudes in hundreds of years was against the scientific consensus---there was no way that Milanković cycles could explain that. Sampling errors seemed more likely, and the idea of rapid climate change was unsettling.
Today we know from ice core samples and more sophisticated ocean sediment sampling techniques that rapid changes in the climate have indeed occurred in the past. And not just in hundreds of years. But in a decade. Or less. Emiliani's work was a big clue that climate was not a stable beast. That climate could turn on a dime. But that realization only came much later.
Harold Urey, 1963:
Cesare Emiliani 1952 (?)
However, not all was quiet on the anthropogenic global warming front. Harold Urey (1893-1981) is one of the towering giants of 20th century science. He won the Nobel Prize for chemistry in 1934 for research in isotopes. He discovered deuterium in 1931, and was the first to isolate pure liquid deuterium from liquid hydrogen. He did much research on the isotopes of uranium, being a member of the brain trust that helped develop the atom (fission) bomb. He came up with a model for the early atmosphere of the Earth in 1952, speculating it was composed of ammonia, methane, and hydrogen, which he published in his book The Planets: Their Origin and Development. Urey's hypothesis for the composition of the atmosphere of the early Earth has since been shown to be wrong, but it was a good kind of wrong that stimulated a lot of research. Harold Urey was Stanley Miller's professor and adviser, and together they crafted the Miller-Urey experiment, one of the most famous experiments of 20th century science. This experiment showed that complex organic compounds, including many amino acids, could be generated easily and in large quantities by natural processes.
Harold Urey was also Isaac Asimov's chemistry professor at Columbia University.
After World War II, Harold Urey turned his attention to isotopes of oxygen. It was a natural question for him to explore. Urey had used centrifuges to separate deuterium from ordinary hydrogen, and had helped work out how to separate uranium-235 from uranium-238 by creating uranium hexafluoride which could be spun in centrifuges to separate the lighter uranium-235 from the heavier uranium-238 (and helped created the more exciting and dangerous world of today)
Harold Urey realized that evaporation of water and condensing it into glaciers could act as a natural way to separate isotopes. Oxygen-18 is a rare but stable isotope of oxygen. A water molecule containing an atom of oxygen-18 is heavier than water molecules containing oxygen-16, and does not evaporate as easily. This means that during ice ages, when more and more evaporated water is trapped in ice sheets, the remaining water in the oceans is enriched in oxygen-18. Therefore, the more oxygen-18 is concentrated in the oceans, the greater the volume of ice sheets and (presumably) the colder the Earth was!
Urey wrote in 1947 that we should check coring samples of the ocean for deposits of foraminifera (forams) shells in the sediments, hypothesizing that those living in times of past ice ages would have enriched levels of oxygen-18 in their shells. This was the first time that nuclear science and biology had been combined to solve a scientific problem!
The problem was taken up by Cesare Emiliani (1922-1995), a geologist from Italy who was one of Urey's students after the war (Many of Urey's students became scientific giants of their own working on problems suggested by Urey).
There were many difficult problems. Sediment coring up until that time was not very sophisticated---it was simply dropping a very heavy and dense metal tube into oceanic sediments. This had been done since the 1870s Challenger expedition, discussed in a previous blog entry, but it blurred and mixed the core samples too much to provide reliable samples of foram shells inside. This is not to say that these primitive core samples were useless---they did provide much information on sediment layers but they were too crude for the sort of research Emiliani was doing.
Borge Kullenberg saved the day. Working on the Swedish Deep Sea Expedition of 1947, he developed a new coring device that used a piston which deployed when the coring tube hit the ocean floor, enabling core sample tubes to be wider and penetrate far deeper into the ocean floor sediments. The coring samples were much clearer, and were 15 meters long, instead of a couple meters. By 1951 he developed a 20 meter coring apparatus.
Back in the lab, people could take precise samples of each layer, tease out a few hundred foram shells, which were then ground and roasted in the presence of pure oxygen-16 gas to form carbon dioxide. The oxygen-18 could then be measured by spinning the carbon dioxide in centrifuges, separating the heavy molecules containing oxygen-18 out.
As Kullenberg's new coring apparatus replaced older coring techniques in a few years, lots of samples became available for inspection. Emiliani used the new technique of carbon dating on the top layers to determine an average rate of sediment deposition on the ocean floor. (beyond about 40,000 years carbon dating does not work as carbon-14 decays). With the new sediment cores of 20 meters, he was able to get samples as much as 300,000 years old.
Emiliani found several pieces of the climate puzzle.
He found that the signature of ice ages could be clearly and consistently seen from ocean-floor sediment samples around the globe.
He also found that the temperature curves generated from these samples matched the Milanković theory very well.
Milanković had not achieved much scientific recognition up until this point---his chronology for ice ages differed from the scientific consensus developed in the late 19th century. But he lived long enough to see vindication through Emiliani's work.
Emiliani also found that there were sharp changes in the temperature of the earth---lots of evidence that ice ages were not smooth curves of cooler and warmer temperatures, but lots of sharp jagged swings in temperature, in periods of hundreds of years. Sharp advances and retreats.
When Emiliani published his research in 1955, it was recognized immediately as groundbreaking. The conclusion that ice ages were driven by Milanković cycles was accepted.
But the sharp temperature swings were not. The coring technique was new--perhaps it was affecting the sampling. The idea that the climate could change by large magnitudes in hundreds of years was against the scientific consensus---there was no way that Milanković cycles could explain that. Sampling errors seemed more likely, and the idea of rapid climate change was unsettling.
Today we know from ice core samples and more sophisticated ocean sediment sampling techniques that rapid changes in the climate have indeed occurred in the past. And not just in hundreds of years. But in a decade. Or less. Emiliani's work was a big clue that climate was not a stable beast. That climate could turn on a dime. But that realization only came much later.
Harold Urey, 1963:
Cesare Emiliani 1952 (?)
Subscribe to:
Posts (Atom)