The pause in global warming research continued a surprisingly long time. World War II accounts for part of this--scientists were working on problems directly related to the war effort and not more esoteric research. Afterward, there was the Cold War, in which although funding for scientific research expanded enormously, much continued to be directed towards military applications. This was the case until the International Geophysical Year of 1957-1958, which stimulated much research relevant to anthropogenic global warming, triggering investigations some of which continue to this day. But that's for another entry.
However, not all was quiet on the anthropogenic global warming front. Harold Urey (1893-1981) is one of the towering giants of 20th century science. He won the Nobel Prize for chemistry in 1934 for research in isotopes. He discovered deuterium in 1931, and was the first to isolate pure liquid deuterium from liquid hydrogen. He did much research on the isotopes of uranium, being a member of the brain trust that helped develop the atom (fission) bomb. He came up with a model for the early atmosphere of the Earth in 1952, speculating it was composed of ammonia, methane, and hydrogen, which he published in his book The Planets: Their Origin and Development. Urey's hypothesis for the composition of the atmosphere of the early Earth has since been shown to be wrong, but it was a good kind of wrong that stimulated a lot of research. Harold Urey was Stanley Miller's professor and adviser, and together they crafted the Miller-Urey experiment, one of the most famous experiments of 20th century science. This experiment showed that complex organic compounds, including many amino acids, could be generated easily and in large quantities by natural processes.
Harold Urey was also Isaac Asimov's chemistry professor at Columbia University.
After World War II, Harold Urey turned his attention to isotopes of oxygen. It was a natural question for him to explore. Urey had used centrifuges to separate deuterium from ordinary hydrogen, and had helped work out how to separate uranium-235 from uranium-238 by creating uranium hexafluoride which could be spun in centrifuges to separate the lighter uranium-235 from the heavier uranium-238 (and helped created the more exciting and dangerous world of today)
Harold Urey realized that evaporation of water and condensing it into glaciers could act as a natural way to separate isotopes. Oxygen-18 is a rare but stable isotope of oxygen. A water molecule containing an atom of oxygen-18 is heavier than water molecules containing oxygen-16, and does not evaporate as easily. This means that during ice ages, when more and more evaporated water is trapped in ice sheets, the remaining water in the oceans is enriched in oxygen-18. Therefore, the more oxygen-18 is concentrated in the oceans, the greater the volume of ice sheets and (presumably) the colder the Earth was!
Urey wrote in 1947 that we should check coring samples of the ocean for deposits of foraminifera (forams) shells in the sediments, hypothesizing that those living in times of past ice ages would have enriched levels of oxygen-18 in their shells. This was the first time that nuclear science and biology had been combined to solve a scientific problem!
The problem was taken up by Cesare Emiliani (1922-1995), a geologist from Italy who was one of Urey's students after the war (Many of Urey's students became scientific giants of their own working on problems suggested by Urey).
There were many difficult problems. Sediment coring up until that time was not very sophisticated---it was simply dropping a very heavy and dense metal tube into oceanic sediments. This had been done since the 1870s Challenger expedition, discussed in a previous blog entry, but it blurred and mixed the core samples too much to provide reliable samples of foram shells inside. This is not to say that these primitive core samples were useless---they did provide much information on sediment layers but they were too crude for the sort of research Emiliani was doing.
Borge Kullenberg saved the day. Working on the Swedish Deep Sea Expedition of 1947, he developed a new coring device that used a piston which deployed when the coring tube hit the ocean floor, enabling core sample tubes to be wider and penetrate far deeper into the ocean floor sediments. The coring samples were much clearer, and were 15 meters long, instead of a couple meters. By 1951 he developed a 20 meter coring apparatus.
Back in the lab, people could take precise samples of each layer, tease out a few hundred foram shells, which were then ground and roasted in the presence of pure oxygen-16 gas to form carbon dioxide. The oxygen-18 could then be measured by spinning the carbon dioxide in centrifuges, separating the heavy molecules containing oxygen-18 out.
As Kullenberg's new coring apparatus replaced older coring techniques in a few years, lots of samples became available for inspection. Emiliani used the new technique of carbon dating on the top layers to determine an average rate of sediment deposition on the ocean floor. (beyond about 40,000 years carbon dating does not work as carbon-14 decays). With the new sediment cores of 20 meters, he was able to get samples as much as 300,000 years old.
Emiliani found several pieces of the climate puzzle.
He found that the signature of ice ages could be clearly and consistently seen from ocean-floor sediment samples around the globe.
He also found that the temperature curves generated from these samples matched the Milanković theory very well.
Milanković had not achieved much scientific recognition up until this point---his chronology for ice ages differed from the scientific consensus developed in the late 19th century. But he lived long enough to see vindication through Emiliani's work.
Emiliani also found that there were sharp changes in the temperature of the earth---lots of evidence that ice ages were not smooth curves of cooler and warmer temperatures, but lots of sharp jagged swings in temperature, in periods of hundreds of years. Sharp advances and retreats.
When Emiliani published his research in 1955, it was recognized immediately as groundbreaking. The conclusion that ice ages were driven by Milanković cycles was accepted.
But the sharp temperature swings were not. The coring technique was new--perhaps it was affecting the sampling. The idea that the climate could change by large magnitudes in hundreds of years was against the scientific consensus---there was no way that Milanković cycles could explain that. Sampling errors seemed more likely, and the idea of rapid climate change was unsettling.
Today we know from ice core samples and more sophisticated ocean sediment sampling techniques that rapid changes in the climate have indeed occurred in the past. And not just in hundreds of years. But in a decade. Or less. Emiliani's work was a big clue that climate was not a stable beast. That climate could turn on a dime. But that realization only came much later.
Harold Urey, 1963:
Cesare Emiliani 1952 (?)
Sunday, March 27, 2011
Saturday, March 26, 2011
Best tsunami video
I've seen a lot of tsunami videos on youtube---and many are very impressive. But this is the best one.
Tuesday, March 15, 2011
A discourse on salinity, early life and the environment of the ancient Earth.
One of the problems with ocean chemistry was that oceanographers were trying to solve the great salinity mystery. And what was that?
Oceanographers had known for a long time that the salt concentration of the oceans could be accounted for by rivers dissolving salt and depositing it in the seas where it concentrates. At the rate of salt deposition by rivers, the oceans were about 80-90 million years old. This had been known since the late 19th century.
And in fact there was another factor. There is an old Norse myth that the reason the oceans are salty is that there is a giant salt mill under the sea, forever churning away. The Norse were not far wrong---the spreading centers at the mid-ocean ridges are continuously putting more salt in the sea. We've all seen those hydrothermal vents---they are full of salt dissolved very efficiently from the hot rocks they percolate through. So the actual time it takes to achieve today's ocean salinity is only 60 million years.
This age for the oceans was supported by Lord Kelvin, who believed that the sun was not much more than 100 million years old, and probably less.
The young salt oceans were used to support evolution (our blood is about 0.8% salt, and it was supposed that this was the salinity of the ocean when our amphibian ancestors left the sea, with that concentration 'preserved' in our bodies till this day (I read this in my 10th grade biology book as well) It was also used to attack evolution because Charles Darwin thought the world had to be several billion years old.
Geological evidence by the 1920s with radioactive decay of uranium into lead proved that the world was over 2 billion years old. The question immediately became, why aren't the oceans more salty?
Equilibrium had long been recognized as the oceans being 15% salt. That's what the salinity would be if there was no way to remove salt from the oceans. That clearly hadn't happened. And had never happened.
Our cells, animal, fish, plant, cannot tolerate that much salt. Of course a 2% salt concentration would kill us very quickly through osmotic dessication. But even if the concentration is even between cell walls and their surrounding fluid, at 5% the 'pulls' of the sodium and chlorine ions dissolved in the water will pull apart the phospholipids that make up the cell membrane.
Yes, there are freaky organisms like brine shrimp that are able to keep salt out of their bodies (their internal salt concentration is the same as ours) Life is still possible with salinity over 5%, but all higher forms of life would go extinct faced with such salinity (aside from oddballs like brine shrimp) and the forms of life would have been very different even if salinity had reached 5%--and such life forms are rarely observed in the fossil record. Also, marine organisms that would have gone extinct if salinity had risen above 5% have not.
This drove oceanographers to distraction. How the hell do oceans get rid of their salt? Salt spray carrying salt back to the land was out. Except for hurricanes, salt spray does not go far inland, and any salt deposited on shore a few yards away washes back in with the next rain. And analysis of rainwater shows that salt is present in the most miniscule amounts.
It has been recognized that salt can be removed through evaporite deposits and covered with sediment that prevents it redissolving into the sea. When the Mediterranean dried up several times 5-6 million years ago each occurrence removed trillions of tons of salt that were covered by river and wind-borne sediments. Salt domes form in similar circumstances, through evaporation from restricted marine basins and burial under sediment.
That was worked out gradually in the 1960s and 1970s, but there is still a nagging problem. That can account for the salinity of the oceans staying well below 5%, but it seems a bit weak to most. After all, what happens if due to some continental configuration, there just aren't any restricted marine basins (or enough of them) to form enough salt deposits to keep ocean salinity down? This salt deposition process can account for the average salinity of the oceans, but with varying continental configurations there would be large and lethal salt variations.
Another mechanism for salt removal is the subduction of oceanic crust, impregnated with salt water in every pore---steam is released in volcanoes, but not much salt (usually---there are a few volcanoes along subduction zones that have very salty magma)
Proponents of GAIA theory propose that ocean salinity is under biological control. They propose two main mechanisms---salt being captured/incorporated into diatom and coccolith shells as they drift to the ocean floor after the animals die. The higher the salinity, the more salt they would capture. This hypothesis was tossed around a lot in the 1970s, but has since been found wanting. Diatoms do not capture more salt in their shells when salinity rises---and at much above 4.5% salinity they die.
The other is that reef building organisms help create restricted basins that trap salt deposits under sediments and marine skeletons. This does have some backing to it--coral atolls do trap layers of salt in lagoons and bury it under coral skeletons, and the Great Barrier Reef could certainly trap many billions of tons of salt.
But even this hypothesis seems weak. Reef building organisms have existed for several hundred million years, with different types of organisms forming them in different periods. But the oceans formed more than 4 billion years ago. So reef-building organisms cannot account for the stability of the salinity of the oceans for over 3 billion years. (Yes there were stromatolites 3 billion years ago. And they do trap salt, to a degree. Quite avidly, actually. But they live in tidal zones, and not in shallow marine seas, where they could trap salt on a much larger scale. Stromatolites simply can't trap enough salt to keep the salinity stable)
There are some negative feedbacks that help stabilize the salinity of the oceans in the absence of biological control. During the time of Pangaea, all the continents were together, and there were no marginal, enclosed seas, or very few compared to today (The Gulf of Mexico, the Red Sea, the Mediterranean Sea, the Persian Gulf, the Caspian Sea, among other examples). But computer modeling and geologic evidence also shows that most of Pangaea was very arid--far away from the oceans and their moisture bearing winds. Much like the interior of Asia, only more so. And large endorheic basins trapped salt carried by what rivers there were in evaporite deposits in the interior of continents. (although these could get washed out later when exposed to rainfall when the continents separated)
So, supercontinent---not many marginal seas to form evaporite deposits, but not much salt transport from the land. Continents scattered about--more rainfall on land but more marginal/partially enclosed seas to trap salt.
But it's still hard to see how there wouldn't be salinity crises occasionally. Until recently, we didn't even know how continents assembled and broke apart very well before Pangaea. But over the last 15 years there has been a revolution in paleocontinent studies---through careful exploration and analysis of isotopes in rocks, geologists believe that they have identified all the prior supercontinents!
It has to be said that supercontinents have been increasing in size as continental crust increases with time. Before Vallbara, there is no evidence for continents at all. Earth seems to have removed its heat through hot spots. 4 billion years ago, if we had seen the Earth we would have seen a planet-wide ocean, probably less than 3% coverage of land, but lots of volcanic island chains, and some island arcs as subduction began. The first "continents" were probably large islands similar in size to Borneo and Madagascar today. That is not to say these were the first "continents"---they were not.
Continental crust is considerably less dense than oceanic crust, and much less dense than the mantle. Granites and other "light" rocks form from differentiation in subduction zones. Less dense minerals stay on the surface of the Earth, and heavier minerals form oceanic crust or remain in the mantle. Continents, including continental shelves, cover almost 40% of the surface of the Earth.
This addition of continental crust, currently a few cubic kilometers a year, has some interesting implications. Assuming that the volume of the oceans has been broadly similar for the past 4 billion years, small, isolated subcontinents would not have had marine continental shelves. A few percent of the surface of the earth in elevated continental crust would have let the oceans "fit" around them. As continents took up more and more surface area, oceans would necessarily shrink in area and become deeper. When continental crust reached a large fraction of the surface of the earth, the oceans would not have as much room, and spread over the lower margins of continents.
Think of it this way. If all the Earth was covered by continental crust, the oceans would still exist and simply cover the lower areas of the continents. There would be less land area than today, with high mountain chains and plateaus like Tibet being the only land areas.
This helps find the solution to a mystery--why did photosynthetic bacteria "wait" over 1.5 billion years to change the atmosphere, releasing enough oxygen to become a part of the atmosphere (oxygen is a very reactive gas). Fossil bacteria from over 2.5 billion years ago have very similar appearances to modern photosynthetic bacteria today. In fact, some may be the same species! Bacteria multiply very quickly---if we found an earthlike planet, devoid of life, with oceans and continents, a similar climate and enough trace elements and minerals that bacteria need, we could seed the planet with bacteria and they would multiply and become ubiquitous in a few years. So why didn't bacteria do that during the Archean era?
The answer may be that until the time of the Great Oxygenation Event there were not enough continents--or more properly, continents covering enough of the surface area of the Earth to have continental shelves. Continental shelves are good environments for bacteria---shelf waters receive minerals and other dissolved solids from wind erosion (dust) and more importantly river erosion. The first continental shelves may have formed 2.5 billion years ago, and triggered a population explosion of bacteria---which were finally able to release oxygen fast enough, and in large enough quantities, to fill up the "oxygen sinks". The primary sink seems to have been dissolved iron in the oceans. In the absence of free oxygen, iron compounds are mostly easily soluble in water. The anoxic oceans of the early Earth were a rich soup of dissolved metal compounds. But in the presence of oxygen, iron forms iron oxides (rust) that are not soluble in water.
The release of large quantities of free oxygen had tremendous effects on the Earth and it's environment. Iron and other metals used by bacteria precipitated out of the oceans to form trillions of tons of banded iron formations that are still important today---most of our iron we mine comes from those formations. Within a brief geological period, minerals precipitated out, and bacteria had to evolve in a new mineral/metal-poor environment, evolving new metabolic pathways to use what iron and other metals they needed more efficiently.
And also with free oxygen present in the oceans, for the first time Eukaryotes evolved. These are more complex cells that have organellesand nuclei--specialized structures better able to deal with the metal-poor seas. It has to be said here that the fossil record of life before large animals and plants evolved is not all we could wish for. Rock formations become increasingly rare beyond 2 billion years ago, and microscopic organisms are difficult to detect. Instead of just looking at a rock outcrop and seeing it packed with trilobites, scientists have to pick rock that they hope will contain microscopic fossils, and inspect the rocks very carefully to find them.
Another difficulty is that almost all old rocks are continental crust. Seafloor subduction eliminates almost all oceanic crust--the oldest oceanic crust on the sea floor is 180 million years old and is about to be subducted under the Philippine Plate. Oceanic crust almost as old is present in the Gulf of Mexico, caught and dragged along by the North American Plate. This may last longer than 180 million years, but still is not helpful for seeing how Archean ocean life evolved.
The earliest eukaryotic organism that we know of is grypania, a form of algae.
Note that higher plants and animals evolved from the same eukaryotic root. Plants did not evolve out of photosynthesizing bacteria, and animals did not evolve from other bacteria. The eukaryotic cell evolved only once, with plants, animals, fungi all springing from one eukaryotic ancestor. Pretty much the life we see.
The differences between eukaryotic cells and prokaryotic cells are fundamental:
A eukaryotic cell:
And a prokaryotic cell:
Notice how a eukaryotic cell has organelles that specialize in different functions. A prokaryotic cell has its nuclear material scattered about, without a discrete nucleus, and lacks specialized internal structures.
Remember that almost all Archean life was marine, in the oceans. Their fossils were subducted billions of years ago. What we know of Archean life comes from two sources---bacterial life on continents in wet areas---lakes or rivers where bacteria could survive on land, or in small areas of oceanic crust that were "caught" by continents as they expanded and collided. Those are the sources of our banded iron formations today---the source of most of our iron and steel.
But with almost all our oceanic crust subducted and long gone in less than 200 million years, what remains of our view of Archean life is very incomplete. Conditions in wetter parts of the early continents were not representative of marine life, and conditions where oceanic crust was about to be caught up and incorporated into continents may not have been representative either.
An illustration of the current age of the oceanic crust, worldwide:
The Great Oxygenation Event had other impacts. The presence of free oxygen is incompatible with large amounts of methane in the atmosphere. Our models show that before the mass release of free oxygen, the Earth's atmosphere had significant quantities of methane. By that I mean about 20 times as much, perhaps 4 ppm in an atmosphere 10 times as thick as ours today. (Methane cannot have been much more abundant than that--if it had been much more the Earth would have been brought up to the boiling point, with all the CO2 present as well. That never happened) Methane (CH4) reacts quickly with oxygen. One CH4 molecule combines with 2 O2 molecules to form two molecules of water (H2O) and one of carbon dioxide (CO2) In short, CH4 + 2(O2) = 2(H20) + 1(CO2) Methane, as we know, is a potent greenhouse gas. The sun was significantly dimmer more than 2 billion years ago, dim enough that an Earth with today's atmospheric composition would be a snowball earth. This is known as the Faint Young Sun Paradox. What seems to have happened is that oxygen accumulated in the seas, precipitating iron and other metals out, until (almost) all metal compounds were precipitated out. What happened next?
Free oxygen then began diffusing out of the oceans into the atmosphere. And all hell broke loose. The Earth's early atmosphere did not resemble today's. If we could go back in time, we would have to wear pressure suits, well shielded from radiation (radioactivity was thousands of times greater than today in the oceans) and be well insulated. Carbon dioxide was superabundant--so much so that the there was 10 times as much atmosphere as today, 10,000 millibars, with about 92% CO2 and 8% N2. It was very warm--geochemical evidence shows that the Earth was 40°C to 50°C. With a near worldwide ocean, it was very humid. Also in the absence of oxygen to react with chemicals in the atmosphere, it was probably very hazy even when clouds were absent. There were no blue skies. The appearance of the Earth may have resembled Titan. Ultraviolet radiation hit the atmosphere, generating photochemical smog, and reached the Earth's surface far more strongly than today (However, the faint sun was cooler, and ultraviolet radiation was even more reduced than visible light, being perhaps 1/3 to 1/2 as much as today. Even so, ultraviolet radiation was far stronger on the earth). Ultraviolet radiation, along with far more prevalent radioactivity, would have created and broken apart compounds far more than we see on the Earth today, with some of those compounds being useful to life. Hostile and alien it may seem to us, but 3 billion years ago life was pretty 'easy' for bacteria. Warm temperatures make chemical reactions go faster and there were plenty of metals dissolved in the oceans for them to use.
The first thing that happened to the early Earth's atmosphere was that as photosynthesis accelerated, carbon dioxide was drawn down. The atmosphere would have become thinner, and temperatures would have begun to fall. Why an ice age wasn't triggered quickly can seem mysterious, but there are two major factors why the early Earth would have been more resistant to ice ages. One was the scarcity of continental land masses, still probably 10% or less of the Earth's surface. There may not have been land masses near the poles to freeze up, accumulate ice, and increase the Earth's albedo. In fact we know there weren't, because otherwise the Earth would have frozen into a Snowball Earth more quickly than it did. When oxygen finally began to accumulate in the atmosphere after precipitating out the iron and other metals in the oceans, it then quickly reacted with the photochemical smog in the atmosphere, clearing it out, and reducing the albedo of the Earth. These two negative feedbacks enabled the Earth to remain warm long enough to allow the Great Oxygenation Event to proceed.
Imagine being on the Earth ~2.5 billion years ago, after millions of years of photosynthesis and when oxygen was just beginning to accumulate. The pressure was down to perhaps 2,000 mb, twice today's pressure, and a mix of roughly equal amounts of CO2 and N2 (nitrogen). Radioactivity in the early oceans was thousands of times greater than today and resembled Deinococcus radiodurans. It may be that the the great radioactive cleanup was the most important factor in the evolution of eukaryotes--bacteria before then had to repair themselves from radiation damage--and more complex cells have more things that can go wrong. We would still need pressure suits, with the pressure twice today's level, and oxygen. We could ditch the radiation shielding.
The Earth still looked very different, under clear skies. The sky was green, not blue (large quantities of CO2 generate a green sky). The Sun was a little smaller, a little more orange. Enough to be recognizably different. But we could see it in good weather. Temperatures were more moderate, perhaps 20°C to 30°C over the Earth. It was a little more like home.
But finally, the oxygen reacting with the CO2, CH4, and carbonyl sulfide (also a potent greenhouse gas) was too much. There were no large continental land masses at the poles, and albedo was decreasing, but the reduction of greenhouse gases finally overcame those negative feedbacks. The Earth descended into the Huronian Glaciation, perhaps the most severe global cooling the Earth ever endured. The global ocean froze from pole to equator, and remained that way for 300 million years, with a few brief breaks.
(The reason for the occasional breaks is that when the oceans froze, interaction between the oceans and seas was mostly cut off. Even though oxygen reacted slowly in a drier, cooler environment, eventually it would get used up. Aside from a little photosynthesis from bacteria in ice near the surface, and in hot springs and near volcanoes, photosynthesis almost stopped. The ice was at least 1 km thick. CO2 and methane would accumulate in the oceans again, and they would become anoxic again. Once in a while, an asteroid or comet, or massive volcanic activity would break up large areas of ice, and the greenhouse gases would bubble up and thaw the world. Until photosynthesis drew down the greenhouse gases, precipitated out the metals in the sea, and cooled the earth again.)
This switching back and forth in the environment from cold to hot, oxygen to anoxic must have sped up the evolution of life greatly. Also, each warm break would have been less and less radioactive---precipitated radioactive compounds on the seafloor and subducted away were not returned. This created an environment more favorable to evolve complex, eukaryotic life.
Finally about 2.1 billion years ago, the snowball earth thawed. Perhaps the slowly brightening sun was enough to thaw the Earth.
It was still not like our Earth. Oxygen was only about 1-2% of the atmosphere. The sky was still green. But the sun was a little brighter, a little less orange. A little closer to home.
The timing of the first continental shelves, generating the first population explosions of bacteria that were enough to change the chemistry of the oceans and atmosphere was fortunate. If continental shelves had formed in large areas 3.5 billion years ago, instead of 2.5 billion years ago, with the even fainter sun the oceans would have frozen right to the sea floor. Life would still have been possible around hydrothermal vents. But the Earth would have frozen so deeply that CO2 would have also frozen out. The temperature would have plunged to -200°F. Chemical reactions at such temperatures would be too slow for life as we know it to operatem and there would be no liquid water. Even when ice is frozen to -50°F or -100°F, mineral contaminants and exposure to the sun can create small mircoscopic pores or films of liquid water for bacteria to live in. But not -200°F. Also, with no ocean present under a thick ice sheet, even 1 km thick, recovery would be far more difficult. The reason is this: If an asteroid or comet hit the oceans, like the Chicxulub impact, even if there was a kilometer-thick layer of ice there was still a lot of liquid water underneath. When an impact broke open a million square kilometers of ice, the ocean underneath would fizz and release its greenhouse gases back. Currents would bring more water to release their gases, and so on. The gases released from currents bringing in new water would keep releasing more greenhouse gases. If the ocean is frozen solid, an impact would release the gases from the ice it melted and vaporized, but not from all the oceans away from the local impact. Also, the extreme cold would cause CO2 to quickly refreeze, making its greenhouse effect very brief. Only the largest impacts could have thawed the Earth. An impact by a 100 mile wide asteroid could have done it---but we know there have been none in the past 3.5 billion years. An impact that big on a thawed Earth would have vaporized all the oceans, raising the Earth to beyond the boiling point. That hasn't happened on our Earth. It could happen on an alternate Earth that had mass photosynthesis develop early from faster-growing continents and their accompanying continental shelves. But it's awfully chancy.
What if the continents had grown more slowly? And the Earth had waited until 1.5 billion years ago to have large continental shelves, and photosynthesis explode? That would have been too late.
The reason is that by about 2.5 billion years ago, the Earth was getting into trouble. On the verge of breaking out into a fever. Even in the absence of oxygen, weathering does take place on continents. CO2 is an acid, and combines with minerals and is removed from the atmosphere. A little of this carbon would sink to the sea floors and be subducted away, although most was consumed by bacteria that generated methane. So CO2 was, very slowly, falling in the atmosphere. But it was not falling fast enough. Many models, although not all say that between 1 and 2 billion years ago the temperature of the earth would have reached the boiling point. And then we would have a runaway greenhouse. The oceans would have become steam. And there would have been no going back. Eventually, ultraviolet dissociation in the upper atmosphere would have dissociated water molecules, allowing hydrogen to escape. The oxygen would combine with carbon and other elements, leading to an Earth like Venus. Dry. Hot. Dead. A bit 'cooler', 600°F, not 900°F. And with a little lower pressure---most estimates of the Earth's geochemistry show that there would not be quite as much CO2 ~60 bars instead of 90 bars. But close enough to be a twin of Venus, and incompatible with life.
It is possible to see life recovering from an early snowball Earth. Life could persist in areas of volcanic activity, with hydrothermal vents. The sun would slowly brighten, and maybe eventually an impact of the right size to thaw the Earth instead of boil it would happen. But from a runaway greenhouse, there is no escape.
And now we go back to the salinity mystery. With the low area for continents and continental shelves absent, how marginal basins could have formed to evaporate water and precipitate salt is a real mystery. The answer is that for before 2.5 billion years ago, we simply don't know how salt was removed. It is very mysterious. If there was an unknown mechanism removing salt then, why isn't this mechanism operating now? Could it be that the oceans were simply very salty back then and salt was gradually drawn down when continental shelves first appeared, along with marginal seas? Maybe, but this hypothesis has strong objections. There are salt loving (or salt-tolerant) bacteria known as halophiles that can handle very high salt concentrations today. Halophiles live in the Great Salt Lake and the Dead Sea, and other similar places. But in today's world, they seem like oddballs, almost parasitic. These bacteria depend on oxygen and other chemicals generated in vast amounts from other bacteria and other organisms. They expend great amounts of energy to keep salt out of their internal structure. Their cell walls are distinct. That could represent adaptation by bacteria that have evolved to tolerate very salty niches in the environment. In fact it almost certainly does.
It is very hard to imagine life developing in such saline water. Even if the concentration inside and outside the cell walls is the same, preventing osmotic dessication, the materials cells use to form themselves fall apart because of the strong ionic charges in highly saline water. Phospholipids fall apart. DNA and RNA are pulled apart. So are many amino acids (although not all of them)And these are really fundamental components of life. It is conceivable that there are other molecules besides DNA and RNA that can encode genetic information and be tolerant of salt. But these compounds, if they exist, have not been identified.
How life would have switched from some non-DNA/RNA genetic architecture to the genetic architecture we know is also mysterious. There is some evidence that genes may have originally developed on RNA molecules and then life switched to DNA. But these are very similar molecules. And how could cells have operated without many of the amino acids our cells use to build proteins and transmit information? And then change to DNA/RNA and amino acids? Such a life form discovered today would be strongly considered to be extraterrestrial.
Halophile bacteria today use DNA and RNA, and the same complement of amino acids we do. Since continents formed, there have always been some areas that are highly saline, like the Dead Sea that I mentioned before. Why hasn't any of such life built on different building blocks survived? (or hasn't been discovered yet, what a possibility!)
In short, it is possible that maybe the Archean oceans were far more salty than today, and that life operated using fundamentally different building blocks. But it is hard to see how life could change it's fundamental components so completely. There is no evidence that this has occurred. So I have to say it is highly unlikely.
It is time to review the supercontinents of the past, and what we know about them.
Vaalbara formed gradually 3.6 to 3.1 billion years ago, broke up 2.8 billion years ago. This "supercontinent" was probably about the size of Australia. It is probable that for most of its existence there were some other continental islets, like New Zealand, roaming around. Evidence for it is found in compatible rock formations in South Africa and northwest Australia. The Australian size estimate can be regarded as a maximum--it may have been considerably smaller, more like Greenland. But we do think this was the first landmass larger than 1 million square miles. Because of the paucity of data, no generally accepted reconstruction of its shape and position has been made.
Ur was a subcontinent that formed ~3 billion years ago, and maintained itself for 2 billion years until it broke up into portions of what is now Asia, South America, Africa and Antarctica ~1 billion years ago. It was not a supercontinent, but deserves a brief mention as a very long lived continental structure. When it joined supercontinents, it broke off as itself without major amputations or additions for 2 billion years. It was originally thought to be the oldest continent until evidence for Vaalbara was discovered and accepted, hence it's name.
Kenorland formed ~2.7 billion years ago, broke up ~2.5 billion years ago. It was the first "full size" continent, being about the size of South America. Kenorland is the first continent for which there is evidence of submerged continental shelves. It seems to have not glued together very tightly, with pieces jostling together or a little apart, with large bays or narrow seas between its components. Kenorland played two crucial roles in the development of life. It was the first to have significant areas of shallow seas and bays that supported dense bacterial populations--large enough to oxygenate the oceans and atmosphere. Paleomagnetic studies show that it formed in low latitudes, but that as it was entering its final breakup ~2.5 billion years ago, it moved to a polar region, and possibly helped trigger the first snowball Earth.
When considering Kenorland, it is important to remember that the Earth was far more geologically active then than it is now. Continents may have moved a foot or more per year, instead of a few inches today. And it seems to have been in large pieces most of the time. Like a group of subcontinents the size of the Arabian Peninsula or Greenland, occasionally welded all together, but mostly traveling together close to each other. Paleomagnetic studies indicate that these subcontinents were close together, and sometimes together.
The time of Kenorland also represents a shift in the Earth's geological behavior. Before Kenorland, the Earth was dominated by large hot spots---think hundreds of island chains like Hawaii, with some hot spots much bigger than that. Continental crust was generated from the lighter mineral 'scum' staying on the surface. But this is a slow and inefficient method for creating continental crust. During the time of Kenorland, the Earth's behavior shifted as hot spots declined, and sea floor spreading and subduction became prevalent. This is not to say that seafloor spreading and subduction did not exist before Kenorland, and hot spot volcanism continues today. But it was around the time of Kenorland that plate tectonics, as we see it today, became dominant.
Subduction is a far more efficient and rapid method for generating continental crust than hot spot volcanism. The Earth has also been cooling since its formation, and as a result is becoming, very slowly, less geologically active. This means that until Kenorland, continental crust formed very very slowly. It was also slowly declining in its rate of formation. Someone observing the Earth 3 billion years ago might have concluded that much more continental crust would never be formed.
However, with the switch to a sea floor spreading/subduction regime, the rate of continental crust formation increased rapidly. There was a major pulse of continental crust formation between ~2.5 billion years ago and ~1.8 billion years ago, with new continental crust forming at ~10 times the rate it had averaged during the previous billion years. Continental crust formation then slowed down considerably (although somewhat faster than before Kenorland) and then there was another pulse of continental crust formation from 700 million years ago to 500 million years ago, along with the continents speeding up to a foot a year or more. The reason for the first pulse of continental crust formation is pretty straightforward. The Earth switched to a predominantly sea floor spreading/subduction mode that was more efficient at creating continental crust. The reason for the second pulse 700 million years ago to 500 million years ago is not clear. There are several different theories, but this blog entry is long enough already. Suffice it to say that there is no one theory that is generally accepted.
Both of these pulses in continental crust formation are associated with snowball Earth episodes and great advances and diversification of life. There is a general feeling that these are all connected, and many theories. Again, no one theory yet has general acceptance.
Columbia / aka Nuna / aka Hudsonland formed 1.9 billion years ago and broke up ~1.5 billion years ago. This was the first real supercontinent, about the size of Eurasia. Columbia is estimated to have been about 12,900 kilometres (8,000 miles) from North to South, and about 4,800 km (3,000 miles) across at its broadest part. The east coast of India was attached to western North America, with southern Australia against western Canada. Most of South America span so that the western edge of modern-day Brazil lined up with eastern North America, forming a continental margin that extended into the southern edge of Scandinavia.
The Columbia supercontinent was probably the first supercontinent to have large scale deserts.
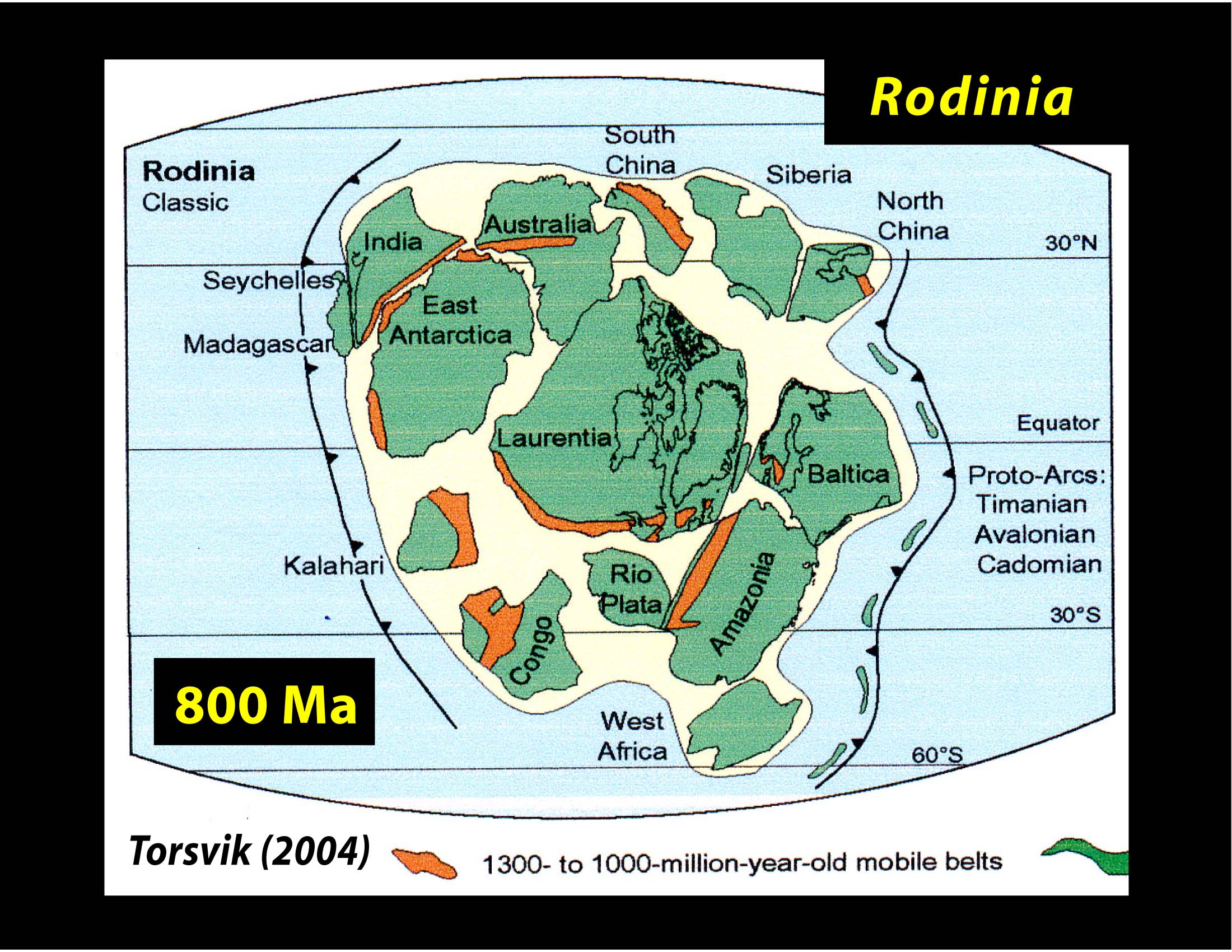
Rodinia 1.1 billion years ago to 750 million years ago. This is the first supercontinent for which we have a consensus of how it fit together. This supercontinent had lots of indentations and marginal seas. Its breakup coincided with the second large scale snowball Earth episode, and the beginning of the evolution of animal life visible to the naked eye. (It is possible that animal life was present earlier--there are fossils of 'worm tracks' which may or may not have been formed by worms over 1 billion years old. These worm tracks could have been produced by non-biological causes. No direct fossil evidence of animal life large enough to see, besides some freakishly big one-cell organisms has been found before the Ediacaran period.
Some evidence from molecular clocks indicates that animal phyla separated as long as 1.5 billion years ago. Others say no, much shorter. It is possible that single-cell or near-microscopic eukaryotic animals separated that long ago, and then a common environmental factor stimulated the growth of animals and plants into larger sizes. We just don't know.
A reconstruction of Rodinia:
Pannotia 600 million years ago (briefly by geologic standards) was a strange supercontinent, that by most reconstructions is shaped like a giant 'V'. For reasons not understood the Earth seems to have had a geological freakout. Continents were flying across the map, bouncing off of each other almost like pinballs, old continental structures that had maintained their integrity through the previous cycles of supercontinental formation and breakup were torn apart, and new continental cores were welded together and remained as one. Continents were moving at 12-18" per year, and the pace of continental crust formation, which had been declining for more than a billion years sped up again. Why all this happened is not clear--there is no consensus yet. Pannotia, which formed the quickest after the breakup of the previous supercontinent, seems to have been almost accidental. The continents whizzing across the Earth happened to meet up, stick together a while, and break apart again. Pannotia only lasted 10-15 million years. After this breakup, with all the reshuffling of the continents, old ones breaking apart and new ones put together we see for the first time some continents that correspond with today's continents.
Pannotia had an unusual configuration. Usually with supercontinents there is a large reduction in seashore length and continental shelf area, but Pannotia was V-shaped (or crescent shaped) with all the continents next to each other in an arc. They were connected but not pressed together. This unique configuration preserved lots of shallow continental shelf areas, over wide latitude zones, with a wide variety of rapidly changing environments that stimulated evolutionary development.
Pannotia's appearance:
For about 50 million years after Pannotia broke up, the continents continued whizzing around. Then around 550 million years ago the continents slowed down and the snowball earth freeze/thaw cycle that operated several times between 750 million years ago and 550 million years ago thawed out decisively. It was almost as if the Earth decided that animal and plant lifeforms had developed, now let them grow!
Another mechanism that undoubtedly stimulated animal evolution was higher levels of oxygen. Before Rodinia, oxygen levels had been rising gradually, so gradually. Oxygen was 2%-3% of the atmosphere 2 billion years ago, and 4-5% 800 million years ago. The only difference is that we would aphyxiate more slowly.
But between Rodinia and Pannotia, when the snowball Earth thawed and the oceans turned green, for the first time oxygen climbed above 5%, spiking to 10%-15%. Oxygen fell again when the oceans froze but kept rebounding. When the Earth thawed definitively, oxygen was 12-15% of the atmosphere---enough for the fist time to support large animals. The reason for this was the subduction of large amounts of carbon during the geological freakout. This allowed oxygen to accumulate from being an important constituent of the atmosphere to a major constituent--from then on in second place. CO2 was down to less than 1%, which was good as the Sun continued to warm. There were fluctuations and extinctions, but never again was oxygen scarcity a dominant global condition (although under certain circumstances, Canfield oceans did form and cause serious problems, such as during the Permian and Cretaceous periods)
The concentration of oxygen during prior geologic periods has been been controversial in some respects. During some periods, it appears that there were very high concentrations of oxygen--30% or more. The problem is that is impossible. The intensity of combustion increases by 70% for each 1% that oxygen increases in the atmosphere. In other words, at 22%, an oxygen fire will generate 70% more energy than at 21%. At 25% sopping wet wood will burn, and at 28%-29% wood will spontaneously ignite. The worlds we read of in science fiction stories with bracing, oxygen-rich atmospheres are fiction indeed. The landing of the spacecraft would incinerate the planet!
This can't be emphasized enough---at 30%, just one lighting strike would trigger a forest fire that would rage across continents.
But yet we find fossils of giant insects like this 30" dragonfly:
These giant insects present a big problem--how could they survive in a 21% oxygen atmosphere like today? They can't. They would asphyxiate almost immediately. The solution is that the atmosphere was 1.5 or 2 times as dense as today---lots of oxygen, in a dense atmosphere for giant insects to metabolize, but with the greater amount of atmosphere keeping oxygen concentrations below 23%.
The problem with this idea is what would the additional gas be? It can't be nitrogen. Nitrogen is the only element on Earth found predominantly in the atmosphere. There isn't much in the oceans, there isn't much in minerals or in soil. If we took all the nitrogen out of the soils and oceans, it would raise nitrogen levels by less that 40%. And nitrogen is needed by life--a big depletion of nitrogen would have reduced life's prevalence drastically in ways not consistent with the fossil record. The giant insects flew in forests full of life. So nitrogen is out.
Carbon dioxide? Nope. Suppose we had an atmosphere of 20% oxygen, 40% nitrogen, and 40% carbon dioxide at double today's pressure. That much CO2 would make it impossible for animals to respire it out of their system. And that is also 2,000 times the amount of CO2 in today's atmosphere. The sun was dimmer, but not that much. By 350 million years ago that much CO2 would send the Earth to the boiling point.
It's hard to see what the mystery gas could be that was added to the atmosphere to dilute oxygen to a non-dangerous level, while keeping oxygen abundant enough for giant insects to thrive.
It has been suggested by some that giant insects could breathe, and had lungs like we do. But there is no fossil evidence for that--the giant dragonflies had spiracles, just like insects today, and received their oxygen by diffusion. There is still a lot of controversy about this!
Here is a summary of the oxygen concentrations during various geologic periods:
Ediacaran 8% with many spikes up and down, first occurrence of 10%+
Cambrian 12.5%
Ordovician 13.5%
Silurian 14%
Devonian 15%
Carboniferous 32.5% (controversial as noted)
Permian 23%
Triassic 16%
Jurassic 26% (controversial)
Cretaceous 30% (controversial)
Paleogene 26% (controversial)
Neogene 21.5%
Quaternary 21%
Pangaea formed 250 million years ago and began breaking up 180-100 million years ago. Pangaea is well known so I will just provide an illustration:
We still don't know about the marginal, partly enclosed seas very well before Pannotia. Salt domes don't last very long by geological standards---they fault, let water in, get subducted, uplifted and eroded--we don't have examples of salt domes or extensive evaporite deposits from billions of years ago.
It has been bandied around that Earth was just very lucky---that we happened to have a continental configuration that at all times kept ocean salinity within reasonable bounds. I have read estimates that the chance of random continental configurations keeping salinity within acceptable ranges for our marine life at less than 1%. I have seen others say less than one in a thousand. Others say that unknown processes could have removed salt more efficiently during the time of the Hadean era, when it was much warmer--salty ocean crust could have been buried by massive hot-spot volcanic eruptions before subduction became dominant--could this have been more efficient at removing/burying salt? We don't know.
Could it be that on most earthlike planets, even when life forms, unlucky continental configurations caused lethal changes in ocean salinity and either extinguished life or kept the biosphere very weak, and at a low level, preventing evolution of more advanced forms?
Nobody knows.
But it was the salinity question that was a big priority of oceanographers in the 1930s and 1940s---why isn't the ocean more salty? That question was the big problem for oceanographers---not solving the trivial mystery of the behavior of carbon dioxide interactions between the atmosphere and ocean.
Oceanographers had known for a long time that the salt concentration of the oceans could be accounted for by rivers dissolving salt and depositing it in the seas where it concentrates. At the rate of salt deposition by rivers, the oceans were about 80-90 million years old. This had been known since the late 19th century.
And in fact there was another factor. There is an old Norse myth that the reason the oceans are salty is that there is a giant salt mill under the sea, forever churning away. The Norse were not far wrong---the spreading centers at the mid-ocean ridges are continuously putting more salt in the sea. We've all seen those hydrothermal vents---they are full of salt dissolved very efficiently from the hot rocks they percolate through. So the actual time it takes to achieve today's ocean salinity is only 60 million years.
This age for the oceans was supported by Lord Kelvin, who believed that the sun was not much more than 100 million years old, and probably less.
The young salt oceans were used to support evolution (our blood is about 0.8% salt, and it was supposed that this was the salinity of the ocean when our amphibian ancestors left the sea, with that concentration 'preserved' in our bodies till this day (I read this in my 10th grade biology book as well) It was also used to attack evolution because Charles Darwin thought the world had to be several billion years old.
Geological evidence by the 1920s with radioactive decay of uranium into lead proved that the world was over 2 billion years old. The question immediately became, why aren't the oceans more salty?
Equilibrium had long been recognized as the oceans being 15% salt. That's what the salinity would be if there was no way to remove salt from the oceans. That clearly hadn't happened. And had never happened.
Our cells, animal, fish, plant, cannot tolerate that much salt. Of course a 2% salt concentration would kill us very quickly through osmotic dessication. But even if the concentration is even between cell walls and their surrounding fluid, at 5% the 'pulls' of the sodium and chlorine ions dissolved in the water will pull apart the phospholipids that make up the cell membrane.
Yes, there are freaky organisms like brine shrimp that are able to keep salt out of their bodies (their internal salt concentration is the same as ours) Life is still possible with salinity over 5%, but all higher forms of life would go extinct faced with such salinity (aside from oddballs like brine shrimp) and the forms of life would have been very different even if salinity had reached 5%--and such life forms are rarely observed in the fossil record. Also, marine organisms that would have gone extinct if salinity had risen above 5% have not.
This drove oceanographers to distraction. How the hell do oceans get rid of their salt? Salt spray carrying salt back to the land was out. Except for hurricanes, salt spray does not go far inland, and any salt deposited on shore a few yards away washes back in with the next rain. And analysis of rainwater shows that salt is present in the most miniscule amounts.
It has been recognized that salt can be removed through evaporite deposits and covered with sediment that prevents it redissolving into the sea. When the Mediterranean dried up several times 5-6 million years ago each occurrence removed trillions of tons of salt that were covered by river and wind-borne sediments. Salt domes form in similar circumstances, through evaporation from restricted marine basins and burial under sediment.
That was worked out gradually in the 1960s and 1970s, but there is still a nagging problem. That can account for the salinity of the oceans staying well below 5%, but it seems a bit weak to most. After all, what happens if due to some continental configuration, there just aren't any restricted marine basins (or enough of them) to form enough salt deposits to keep ocean salinity down? This salt deposition process can account for the average salinity of the oceans, but with varying continental configurations there would be large and lethal salt variations.
Another mechanism for salt removal is the subduction of oceanic crust, impregnated with salt water in every pore---steam is released in volcanoes, but not much salt (usually---there are a few volcanoes along subduction zones that have very salty magma)
Proponents of GAIA theory propose that ocean salinity is under biological control. They propose two main mechanisms---salt being captured/incorporated into diatom and coccolith shells as they drift to the ocean floor after the animals die. The higher the salinity, the more salt they would capture. This hypothesis was tossed around a lot in the 1970s, but has since been found wanting. Diatoms do not capture more salt in their shells when salinity rises---and at much above 4.5% salinity they die.
The other is that reef building organisms help create restricted basins that trap salt deposits under sediments and marine skeletons. This does have some backing to it--coral atolls do trap layers of salt in lagoons and bury it under coral skeletons, and the Great Barrier Reef could certainly trap many billions of tons of salt.
But even this hypothesis seems weak. Reef building organisms have existed for several hundred million years, with different types of organisms forming them in different periods. But the oceans formed more than 4 billion years ago. So reef-building organisms cannot account for the stability of the salinity of the oceans for over 3 billion years. (Yes there were stromatolites 3 billion years ago. And they do trap salt, to a degree. Quite avidly, actually. But they live in tidal zones, and not in shallow marine seas, where they could trap salt on a much larger scale. Stromatolites simply can't trap enough salt to keep the salinity stable)
There are some negative feedbacks that help stabilize the salinity of the oceans in the absence of biological control. During the time of Pangaea, all the continents were together, and there were no marginal, enclosed seas, or very few compared to today (The Gulf of Mexico, the Red Sea, the Mediterranean Sea, the Persian Gulf, the Caspian Sea, among other examples). But computer modeling and geologic evidence also shows that most of Pangaea was very arid--far away from the oceans and their moisture bearing winds. Much like the interior of Asia, only more so. And large endorheic basins trapped salt carried by what rivers there were in evaporite deposits in the interior of continents. (although these could get washed out later when exposed to rainfall when the continents separated)
So, supercontinent---not many marginal seas to form evaporite deposits, but not much salt transport from the land. Continents scattered about--more rainfall on land but more marginal/partially enclosed seas to trap salt.
But it's still hard to see how there wouldn't be salinity crises occasionally. Until recently, we didn't even know how continents assembled and broke apart very well before Pangaea. But over the last 15 years there has been a revolution in paleocontinent studies---through careful exploration and analysis of isotopes in rocks, geologists believe that they have identified all the prior supercontinents!
It has to be said that supercontinents have been increasing in size as continental crust increases with time. Before Vallbara, there is no evidence for continents at all. Earth seems to have removed its heat through hot spots. 4 billion years ago, if we had seen the Earth we would have seen a planet-wide ocean, probably less than 3% coverage of land, but lots of volcanic island chains, and some island arcs as subduction began. The first "continents" were probably large islands similar in size to Borneo and Madagascar today. That is not to say these were the first "continents"---they were not.
Continental crust is considerably less dense than oceanic crust, and much less dense than the mantle. Granites and other "light" rocks form from differentiation in subduction zones. Less dense minerals stay on the surface of the Earth, and heavier minerals form oceanic crust or remain in the mantle. Continents, including continental shelves, cover almost 40% of the surface of the Earth.
This addition of continental crust, currently a few cubic kilometers a year, has some interesting implications. Assuming that the volume of the oceans has been broadly similar for the past 4 billion years, small, isolated subcontinents would not have had marine continental shelves. A few percent of the surface of the earth in elevated continental crust would have let the oceans "fit" around them. As continents took up more and more surface area, oceans would necessarily shrink in area and become deeper. When continental crust reached a large fraction of the surface of the earth, the oceans would not have as much room, and spread over the lower margins of continents.
Think of it this way. If all the Earth was covered by continental crust, the oceans would still exist and simply cover the lower areas of the continents. There would be less land area than today, with high mountain chains and plateaus like Tibet being the only land areas.
This helps find the solution to a mystery--why did photosynthetic bacteria "wait" over 1.5 billion years to change the atmosphere, releasing enough oxygen to become a part of the atmosphere (oxygen is a very reactive gas). Fossil bacteria from over 2.5 billion years ago have very similar appearances to modern photosynthetic bacteria today. In fact, some may be the same species! Bacteria multiply very quickly---if we found an earthlike planet, devoid of life, with oceans and continents, a similar climate and enough trace elements and minerals that bacteria need, we could seed the planet with bacteria and they would multiply and become ubiquitous in a few years. So why didn't bacteria do that during the Archean era?
The answer may be that until the time of the Great Oxygenation Event there were not enough continents--or more properly, continents covering enough of the surface area of the Earth to have continental shelves. Continental shelves are good environments for bacteria---shelf waters receive minerals and other dissolved solids from wind erosion (dust) and more importantly river erosion. The first continental shelves may have formed 2.5 billion years ago, and triggered a population explosion of bacteria---which were finally able to release oxygen fast enough, and in large enough quantities, to fill up the "oxygen sinks". The primary sink seems to have been dissolved iron in the oceans. In the absence of free oxygen, iron compounds are mostly easily soluble in water. The anoxic oceans of the early Earth were a rich soup of dissolved metal compounds. But in the presence of oxygen, iron forms iron oxides (rust) that are not soluble in water.
The release of large quantities of free oxygen had tremendous effects on the Earth and it's environment. Iron and other metals used by bacteria precipitated out of the oceans to form trillions of tons of banded iron formations that are still important today---most of our iron we mine comes from those formations. Within a brief geological period, minerals precipitated out, and bacteria had to evolve in a new mineral/metal-poor environment, evolving new metabolic pathways to use what iron and other metals they needed more efficiently.
And also with free oxygen present in the oceans, for the first time Eukaryotes evolved. These are more complex cells that have organellesand nuclei--specialized structures better able to deal with the metal-poor seas. It has to be said here that the fossil record of life before large animals and plants evolved is not all we could wish for. Rock formations become increasingly rare beyond 2 billion years ago, and microscopic organisms are difficult to detect. Instead of just looking at a rock outcrop and seeing it packed with trilobites, scientists have to pick rock that they hope will contain microscopic fossils, and inspect the rocks very carefully to find them.
Another difficulty is that almost all old rocks are continental crust. Seafloor subduction eliminates almost all oceanic crust--the oldest oceanic crust on the sea floor is 180 million years old and is about to be subducted under the Philippine Plate. Oceanic crust almost as old is present in the Gulf of Mexico, caught and dragged along by the North American Plate. This may last longer than 180 million years, but still is not helpful for seeing how Archean ocean life evolved.
The earliest eukaryotic organism that we know of is grypania, a form of algae.
Note that higher plants and animals evolved from the same eukaryotic root. Plants did not evolve out of photosynthesizing bacteria, and animals did not evolve from other bacteria. The eukaryotic cell evolved only once, with plants, animals, fungi all springing from one eukaryotic ancestor. Pretty much the life we see.
The differences between eukaryotic cells and prokaryotic cells are fundamental:
A eukaryotic cell:
And a prokaryotic cell:
Notice how a eukaryotic cell has organelles that specialize in different functions. A prokaryotic cell has its nuclear material scattered about, without a discrete nucleus, and lacks specialized internal structures.
Remember that almost all Archean life was marine, in the oceans. Their fossils were subducted billions of years ago. What we know of Archean life comes from two sources---bacterial life on continents in wet areas---lakes or rivers where bacteria could survive on land, or in small areas of oceanic crust that were "caught" by continents as they expanded and collided. Those are the sources of our banded iron formations today---the source of most of our iron and steel.
But with almost all our oceanic crust subducted and long gone in less than 200 million years, what remains of our view of Archean life is very incomplete. Conditions in wetter parts of the early continents were not representative of marine life, and conditions where oceanic crust was about to be caught up and incorporated into continents may not have been representative either.
An illustration of the current age of the oceanic crust, worldwide:
The Great Oxygenation Event had other impacts. The presence of free oxygen is incompatible with large amounts of methane in the atmosphere. Our models show that before the mass release of free oxygen, the Earth's atmosphere had significant quantities of methane. By that I mean about 20 times as much, perhaps 4 ppm in an atmosphere 10 times as thick as ours today. (Methane cannot have been much more abundant than that--if it had been much more the Earth would have been brought up to the boiling point, with all the CO2 present as well. That never happened) Methane (CH4) reacts quickly with oxygen. One CH4 molecule combines with 2 O2 molecules to form two molecules of water (H2O) and one of carbon dioxide (CO2) In short, CH4 + 2(O2) = 2(H20) + 1(CO2) Methane, as we know, is a potent greenhouse gas. The sun was significantly dimmer more than 2 billion years ago, dim enough that an Earth with today's atmospheric composition would be a snowball earth. This is known as the Faint Young Sun Paradox. What seems to have happened is that oxygen accumulated in the seas, precipitating iron and other metals out, until (almost) all metal compounds were precipitated out. What happened next?
Free oxygen then began diffusing out of the oceans into the atmosphere. And all hell broke loose. The Earth's early atmosphere did not resemble today's. If we could go back in time, we would have to wear pressure suits, well shielded from radiation (radioactivity was thousands of times greater than today in the oceans) and be well insulated. Carbon dioxide was superabundant--so much so that the there was 10 times as much atmosphere as today, 10,000 millibars, with about 92% CO2 and 8% N2. It was very warm--geochemical evidence shows that the Earth was 40°C to 50°C. With a near worldwide ocean, it was very humid. Also in the absence of oxygen to react with chemicals in the atmosphere, it was probably very hazy even when clouds were absent. There were no blue skies. The appearance of the Earth may have resembled Titan. Ultraviolet radiation hit the atmosphere, generating photochemical smog, and reached the Earth's surface far more strongly than today (However, the faint sun was cooler, and ultraviolet radiation was even more reduced than visible light, being perhaps 1/3 to 1/2 as much as today. Even so, ultraviolet radiation was far stronger on the earth). Ultraviolet radiation, along with far more prevalent radioactivity, would have created and broken apart compounds far more than we see on the Earth today, with some of those compounds being useful to life. Hostile and alien it may seem to us, but 3 billion years ago life was pretty 'easy' for bacteria. Warm temperatures make chemical reactions go faster and there were plenty of metals dissolved in the oceans for them to use.
The first thing that happened to the early Earth's atmosphere was that as photosynthesis accelerated, carbon dioxide was drawn down. The atmosphere would have become thinner, and temperatures would have begun to fall. Why an ice age wasn't triggered quickly can seem mysterious, but there are two major factors why the early Earth would have been more resistant to ice ages. One was the scarcity of continental land masses, still probably 10% or less of the Earth's surface. There may not have been land masses near the poles to freeze up, accumulate ice, and increase the Earth's albedo. In fact we know there weren't, because otherwise the Earth would have frozen into a Snowball Earth more quickly than it did. When oxygen finally began to accumulate in the atmosphere after precipitating out the iron and other metals in the oceans, it then quickly reacted with the photochemical smog in the atmosphere, clearing it out, and reducing the albedo of the Earth. These two negative feedbacks enabled the Earth to remain warm long enough to allow the Great Oxygenation Event to proceed.
Imagine being on the Earth ~2.5 billion years ago, after millions of years of photosynthesis and when oxygen was just beginning to accumulate. The pressure was down to perhaps 2,000 mb, twice today's pressure, and a mix of roughly equal amounts of CO2 and N2 (nitrogen). Radioactivity in the early oceans was thousands of times greater than today and resembled Deinococcus radiodurans. It may be that the the great radioactive cleanup was the most important factor in the evolution of eukaryotes--bacteria before then had to repair themselves from radiation damage--and more complex cells have more things that can go wrong. We would still need pressure suits, with the pressure twice today's level, and oxygen. We could ditch the radiation shielding.
The Earth still looked very different, under clear skies. The sky was green, not blue (large quantities of CO2 generate a green sky). The Sun was a little smaller, a little more orange. Enough to be recognizably different. But we could see it in good weather. Temperatures were more moderate, perhaps 20°C to 30°C over the Earth. It was a little more like home.
But finally, the oxygen reacting with the CO2, CH4, and carbonyl sulfide (also a potent greenhouse gas) was too much. There were no large continental land masses at the poles, and albedo was decreasing, but the reduction of greenhouse gases finally overcame those negative feedbacks. The Earth descended into the Huronian Glaciation, perhaps the most severe global cooling the Earth ever endured. The global ocean froze from pole to equator, and remained that way for 300 million years, with a few brief breaks.
(The reason for the occasional breaks is that when the oceans froze, interaction between the oceans and seas was mostly cut off. Even though oxygen reacted slowly in a drier, cooler environment, eventually it would get used up. Aside from a little photosynthesis from bacteria in ice near the surface, and in hot springs and near volcanoes, photosynthesis almost stopped. The ice was at least 1 km thick. CO2 and methane would accumulate in the oceans again, and they would become anoxic again. Once in a while, an asteroid or comet, or massive volcanic activity would break up large areas of ice, and the greenhouse gases would bubble up and thaw the world. Until photosynthesis drew down the greenhouse gases, precipitated out the metals in the sea, and cooled the earth again.)
This switching back and forth in the environment from cold to hot, oxygen to anoxic must have sped up the evolution of life greatly. Also, each warm break would have been less and less radioactive---precipitated radioactive compounds on the seafloor and subducted away were not returned. This created an environment more favorable to evolve complex, eukaryotic life.
Finally about 2.1 billion years ago, the snowball earth thawed. Perhaps the slowly brightening sun was enough to thaw the Earth.
It was still not like our Earth. Oxygen was only about 1-2% of the atmosphere. The sky was still green. But the sun was a little brighter, a little less orange. A little closer to home.
The timing of the first continental shelves, generating the first population explosions of bacteria that were enough to change the chemistry of the oceans and atmosphere was fortunate. If continental shelves had formed in large areas 3.5 billion years ago, instead of 2.5 billion years ago, with the even fainter sun the oceans would have frozen right to the sea floor. Life would still have been possible around hydrothermal vents. But the Earth would have frozen so deeply that CO2 would have also frozen out. The temperature would have plunged to -200°F. Chemical reactions at such temperatures would be too slow for life as we know it to operatem and there would be no liquid water. Even when ice is frozen to -50°F or -100°F, mineral contaminants and exposure to the sun can create small mircoscopic pores or films of liquid water for bacteria to live in. But not -200°F. Also, with no ocean present under a thick ice sheet, even 1 km thick, recovery would be far more difficult. The reason is this: If an asteroid or comet hit the oceans, like the Chicxulub impact, even if there was a kilometer-thick layer of ice there was still a lot of liquid water underneath. When an impact broke open a million square kilometers of ice, the ocean underneath would fizz and release its greenhouse gases back. Currents would bring more water to release their gases, and so on. The gases released from currents bringing in new water would keep releasing more greenhouse gases. If the ocean is frozen solid, an impact would release the gases from the ice it melted and vaporized, but not from all the oceans away from the local impact. Also, the extreme cold would cause CO2 to quickly refreeze, making its greenhouse effect very brief. Only the largest impacts could have thawed the Earth. An impact by a 100 mile wide asteroid could have done it---but we know there have been none in the past 3.5 billion years. An impact that big on a thawed Earth would have vaporized all the oceans, raising the Earth to beyond the boiling point. That hasn't happened on our Earth. It could happen on an alternate Earth that had mass photosynthesis develop early from faster-growing continents and their accompanying continental shelves. But it's awfully chancy.
What if the continents had grown more slowly? And the Earth had waited until 1.5 billion years ago to have large continental shelves, and photosynthesis explode? That would have been too late.
The reason is that by about 2.5 billion years ago, the Earth was getting into trouble. On the verge of breaking out into a fever. Even in the absence of oxygen, weathering does take place on continents. CO2 is an acid, and combines with minerals and is removed from the atmosphere. A little of this carbon would sink to the sea floors and be subducted away, although most was consumed by bacteria that generated methane. So CO2 was, very slowly, falling in the atmosphere. But it was not falling fast enough. Many models, although not all say that between 1 and 2 billion years ago the temperature of the earth would have reached the boiling point. And then we would have a runaway greenhouse. The oceans would have become steam. And there would have been no going back. Eventually, ultraviolet dissociation in the upper atmosphere would have dissociated water molecules, allowing hydrogen to escape. The oxygen would combine with carbon and other elements, leading to an Earth like Venus. Dry. Hot. Dead. A bit 'cooler', 600°F, not 900°F. And with a little lower pressure---most estimates of the Earth's geochemistry show that there would not be quite as much CO2 ~60 bars instead of 90 bars. But close enough to be a twin of Venus, and incompatible with life.
It is possible to see life recovering from an early snowball Earth. Life could persist in areas of volcanic activity, with hydrothermal vents. The sun would slowly brighten, and maybe eventually an impact of the right size to thaw the Earth instead of boil it would happen. But from a runaway greenhouse, there is no escape.
And now we go back to the salinity mystery. With the low area for continents and continental shelves absent, how marginal basins could have formed to evaporate water and precipitate salt is a real mystery. The answer is that for before 2.5 billion years ago, we simply don't know how salt was removed. It is very mysterious. If there was an unknown mechanism removing salt then, why isn't this mechanism operating now? Could it be that the oceans were simply very salty back then and salt was gradually drawn down when continental shelves first appeared, along with marginal seas? Maybe, but this hypothesis has strong objections. There are salt loving (or salt-tolerant) bacteria known as halophiles that can handle very high salt concentrations today. Halophiles live in the Great Salt Lake and the Dead Sea, and other similar places. But in today's world, they seem like oddballs, almost parasitic. These bacteria depend on oxygen and other chemicals generated in vast amounts from other bacteria and other organisms. They expend great amounts of energy to keep salt out of their internal structure. Their cell walls are distinct. That could represent adaptation by bacteria that have evolved to tolerate very salty niches in the environment. In fact it almost certainly does.
It is very hard to imagine life developing in such saline water. Even if the concentration inside and outside the cell walls is the same, preventing osmotic dessication, the materials cells use to form themselves fall apart because of the strong ionic charges in highly saline water. Phospholipids fall apart. DNA and RNA are pulled apart. So are many amino acids (although not all of them)And these are really fundamental components of life. It is conceivable that there are other molecules besides DNA and RNA that can encode genetic information and be tolerant of salt. But these compounds, if they exist, have not been identified.
How life would have switched from some non-DNA/RNA genetic architecture to the genetic architecture we know is also mysterious. There is some evidence that genes may have originally developed on RNA molecules and then life switched to DNA. But these are very similar molecules. And how could cells have operated without many of the amino acids our cells use to build proteins and transmit information? And then change to DNA/RNA and amino acids? Such a life form discovered today would be strongly considered to be extraterrestrial.
Halophile bacteria today use DNA and RNA, and the same complement of amino acids we do. Since continents formed, there have always been some areas that are highly saline, like the Dead Sea that I mentioned before. Why hasn't any of such life built on different building blocks survived? (or hasn't been discovered yet, what a possibility!)
In short, it is possible that maybe the Archean oceans were far more salty than today, and that life operated using fundamentally different building blocks. But it is hard to see how life could change it's fundamental components so completely. There is no evidence that this has occurred. So I have to say it is highly unlikely.
It is time to review the supercontinents of the past, and what we know about them.
Vaalbara formed gradually 3.6 to 3.1 billion years ago, broke up 2.8 billion years ago. This "supercontinent" was probably about the size of Australia. It is probable that for most of its existence there were some other continental islets, like New Zealand, roaming around. Evidence for it is found in compatible rock formations in South Africa and northwest Australia. The Australian size estimate can be regarded as a maximum--it may have been considerably smaller, more like Greenland. But we do think this was the first landmass larger than 1 million square miles. Because of the paucity of data, no generally accepted reconstruction of its shape and position has been made.
Ur was a subcontinent that formed ~3 billion years ago, and maintained itself for 2 billion years until it broke up into portions of what is now Asia, South America, Africa and Antarctica ~1 billion years ago. It was not a supercontinent, but deserves a brief mention as a very long lived continental structure. When it joined supercontinents, it broke off as itself without major amputations or additions for 2 billion years. It was originally thought to be the oldest continent until evidence for Vaalbara was discovered and accepted, hence it's name.
Kenorland formed ~2.7 billion years ago, broke up ~2.5 billion years ago. It was the first "full size" continent, being about the size of South America. Kenorland is the first continent for which there is evidence of submerged continental shelves. It seems to have not glued together very tightly, with pieces jostling together or a little apart, with large bays or narrow seas between its components. Kenorland played two crucial roles in the development of life. It was the first to have significant areas of shallow seas and bays that supported dense bacterial populations--large enough to oxygenate the oceans and atmosphere. Paleomagnetic studies show that it formed in low latitudes, but that as it was entering its final breakup ~2.5 billion years ago, it moved to a polar region, and possibly helped trigger the first snowball Earth.
When considering Kenorland, it is important to remember that the Earth was far more geologically active then than it is now. Continents may have moved a foot or more per year, instead of a few inches today. And it seems to have been in large pieces most of the time. Like a group of subcontinents the size of the Arabian Peninsula or Greenland, occasionally welded all together, but mostly traveling together close to each other. Paleomagnetic studies indicate that these subcontinents were close together, and sometimes together.
The time of Kenorland also represents a shift in the Earth's geological behavior. Before Kenorland, the Earth was dominated by large hot spots---think hundreds of island chains like Hawaii, with some hot spots much bigger than that. Continental crust was generated from the lighter mineral 'scum' staying on the surface. But this is a slow and inefficient method for creating continental crust. During the time of Kenorland, the Earth's behavior shifted as hot spots declined, and sea floor spreading and subduction became prevalent. This is not to say that seafloor spreading and subduction did not exist before Kenorland, and hot spot volcanism continues today. But it was around the time of Kenorland that plate tectonics, as we see it today, became dominant.
Subduction is a far more efficient and rapid method for generating continental crust than hot spot volcanism. The Earth has also been cooling since its formation, and as a result is becoming, very slowly, less geologically active. This means that until Kenorland, continental crust formed very very slowly. It was also slowly declining in its rate of formation. Someone observing the Earth 3 billion years ago might have concluded that much more continental crust would never be formed.
However, with the switch to a sea floor spreading/subduction regime, the rate of continental crust formation increased rapidly. There was a major pulse of continental crust formation between ~2.5 billion years ago and ~1.8 billion years ago, with new continental crust forming at ~10 times the rate it had averaged during the previous billion years. Continental crust formation then slowed down considerably (although somewhat faster than before Kenorland) and then there was another pulse of continental crust formation from 700 million years ago to 500 million years ago, along with the continents speeding up to a foot a year or more. The reason for the first pulse of continental crust formation is pretty straightforward. The Earth switched to a predominantly sea floor spreading/subduction mode that was more efficient at creating continental crust. The reason for the second pulse 700 million years ago to 500 million years ago is not clear. There are several different theories, but this blog entry is long enough already. Suffice it to say that there is no one theory that is generally accepted.
Both of these pulses in continental crust formation are associated with snowball Earth episodes and great advances and diversification of life. There is a general feeling that these are all connected, and many theories. Again, no one theory yet has general acceptance.
Columbia / aka Nuna / aka Hudsonland formed 1.9 billion years ago and broke up ~1.5 billion years ago. This was the first real supercontinent, about the size of Eurasia. Columbia is estimated to have been about 12,900 kilometres (8,000 miles) from North to South, and about 4,800 km (3,000 miles) across at its broadest part. The east coast of India was attached to western North America, with southern Australia against western Canada. Most of South America span so that the western edge of modern-day Brazil lined up with eastern North America, forming a continental margin that extended into the southern edge of Scandinavia.
The Columbia supercontinent was probably the first supercontinent to have large scale deserts.
Rodinia 1.1 billion years ago to 750 million years ago. This is the first supercontinent for which we have a consensus of how it fit together. This supercontinent had lots of indentations and marginal seas. Its breakup coincided with the second large scale snowball Earth episode, and the beginning of the evolution of animal life visible to the naked eye. (It is possible that animal life was present earlier--there are fossils of 'worm tracks' which may or may not have been formed by worms over 1 billion years old. These worm tracks could have been produced by non-biological causes. No direct fossil evidence of animal life large enough to see, besides some freakishly big one-cell organisms has been found before the Ediacaran period.
Some evidence from molecular clocks indicates that animal phyla separated as long as 1.5 billion years ago. Others say no, much shorter. It is possible that single-cell or near-microscopic eukaryotic animals separated that long ago, and then a common environmental factor stimulated the growth of animals and plants into larger sizes. We just don't know.
A reconstruction of Rodinia:
Pannotia 600 million years ago (briefly by geologic standards) was a strange supercontinent, that by most reconstructions is shaped like a giant 'V'. For reasons not understood the Earth seems to have had a geological freakout. Continents were flying across the map, bouncing off of each other almost like pinballs, old continental structures that had maintained their integrity through the previous cycles of supercontinental formation and breakup were torn apart, and new continental cores were welded together and remained as one. Continents were moving at 12-18" per year, and the pace of continental crust formation, which had been declining for more than a billion years sped up again. Why all this happened is not clear--there is no consensus yet. Pannotia, which formed the quickest after the breakup of the previous supercontinent, seems to have been almost accidental. The continents whizzing across the Earth happened to meet up, stick together a while, and break apart again. Pannotia only lasted 10-15 million years. After this breakup, with all the reshuffling of the continents, old ones breaking apart and new ones put together we see for the first time some continents that correspond with today's continents.
Pannotia had an unusual configuration. Usually with supercontinents there is a large reduction in seashore length and continental shelf area, but Pannotia was V-shaped (or crescent shaped) with all the continents next to each other in an arc. They were connected but not pressed together. This unique configuration preserved lots of shallow continental shelf areas, over wide latitude zones, with a wide variety of rapidly changing environments that stimulated evolutionary development.
Pannotia's appearance:
For about 50 million years after Pannotia broke up, the continents continued whizzing around. Then around 550 million years ago the continents slowed down and the snowball earth freeze/thaw cycle that operated several times between 750 million years ago and 550 million years ago thawed out decisively. It was almost as if the Earth decided that animal and plant lifeforms had developed, now let them grow!
Another mechanism that undoubtedly stimulated animal evolution was higher levels of oxygen. Before Rodinia, oxygen levels had been rising gradually, so gradually. Oxygen was 2%-3% of the atmosphere 2 billion years ago, and 4-5% 800 million years ago. The only difference is that we would aphyxiate more slowly.
But between Rodinia and Pannotia, when the snowball Earth thawed and the oceans turned green, for the first time oxygen climbed above 5%, spiking to 10%-15%. Oxygen fell again when the oceans froze but kept rebounding. When the Earth thawed definitively, oxygen was 12-15% of the atmosphere---enough for the fist time to support large animals. The reason for this was the subduction of large amounts of carbon during the geological freakout. This allowed oxygen to accumulate from being an important constituent of the atmosphere to a major constituent--from then on in second place. CO2 was down to less than 1%, which was good as the Sun continued to warm. There were fluctuations and extinctions, but never again was oxygen scarcity a dominant global condition (although under certain circumstances, Canfield oceans did form and cause serious problems, such as during the Permian and Cretaceous periods)
The concentration of oxygen during prior geologic periods has been been controversial in some respects. During some periods, it appears that there were very high concentrations of oxygen--30% or more. The problem is that is impossible. The intensity of combustion increases by 70% for each 1% that oxygen increases in the atmosphere. In other words, at 22%, an oxygen fire will generate 70% more energy than at 21%. At 25% sopping wet wood will burn, and at 28%-29% wood will spontaneously ignite. The worlds we read of in science fiction stories with bracing, oxygen-rich atmospheres are fiction indeed. The landing of the spacecraft would incinerate the planet!
This can't be emphasized enough---at 30%, just one lighting strike would trigger a forest fire that would rage across continents.
But yet we find fossils of giant insects like this 30" dragonfly:
These giant insects present a big problem--how could they survive in a 21% oxygen atmosphere like today? They can't. They would asphyxiate almost immediately. The solution is that the atmosphere was 1.5 or 2 times as dense as today---lots of oxygen, in a dense atmosphere for giant insects to metabolize, but with the greater amount of atmosphere keeping oxygen concentrations below 23%.
The problem with this idea is what would the additional gas be? It can't be nitrogen. Nitrogen is the only element on Earth found predominantly in the atmosphere. There isn't much in the oceans, there isn't much in minerals or in soil. If we took all the nitrogen out of the soils and oceans, it would raise nitrogen levels by less that 40%. And nitrogen is needed by life--a big depletion of nitrogen would have reduced life's prevalence drastically in ways not consistent with the fossil record. The giant insects flew in forests full of life. So nitrogen is out.
Carbon dioxide? Nope. Suppose we had an atmosphere of 20% oxygen, 40% nitrogen, and 40% carbon dioxide at double today's pressure. That much CO2 would make it impossible for animals to respire it out of their system. And that is also 2,000 times the amount of CO2 in today's atmosphere. The sun was dimmer, but not that much. By 350 million years ago that much CO2 would send the Earth to the boiling point.
It's hard to see what the mystery gas could be that was added to the atmosphere to dilute oxygen to a non-dangerous level, while keeping oxygen abundant enough for giant insects to thrive.
It has been suggested by some that giant insects could breathe, and had lungs like we do. But there is no fossil evidence for that--the giant dragonflies had spiracles, just like insects today, and received their oxygen by diffusion. There is still a lot of controversy about this!
Here is a summary of the oxygen concentrations during various geologic periods:
Ediacaran 8% with many spikes up and down, first occurrence of 10%+
Cambrian 12.5%
Ordovician 13.5%
Silurian 14%
Devonian 15%
Carboniferous 32.5% (controversial as noted)
Permian 23%
Triassic 16%
Jurassic 26% (controversial)
Cretaceous 30% (controversial)
Paleogene 26% (controversial)
Neogene 21.5%
Quaternary 21%
Pangaea formed 250 million years ago and began breaking up 180-100 million years ago. Pangaea is well known so I will just provide an illustration:
We still don't know about the marginal, partly enclosed seas very well before Pannotia. Salt domes don't last very long by geological standards---they fault, let water in, get subducted, uplifted and eroded--we don't have examples of salt domes or extensive evaporite deposits from billions of years ago.
It has been bandied around that Earth was just very lucky---that we happened to have a continental configuration that at all times kept ocean salinity within reasonable bounds. I have read estimates that the chance of random continental configurations keeping salinity within acceptable ranges for our marine life at less than 1%. I have seen others say less than one in a thousand. Others say that unknown processes could have removed salt more efficiently during the time of the Hadean era, when it was much warmer--salty ocean crust could have been buried by massive hot-spot volcanic eruptions before subduction became dominant--could this have been more efficient at removing/burying salt? We don't know.
Could it be that on most earthlike planets, even when life forms, unlucky continental configurations caused lethal changes in ocean salinity and either extinguished life or kept the biosphere very weak, and at a low level, preventing evolution of more advanced forms?
Nobody knows.
But it was the salinity question that was a big priority of oceanographers in the 1930s and 1940s---why isn't the ocean more salty? That question was the big problem for oceanographers---not solving the trivial mystery of the behavior of carbon dioxide interactions between the atmosphere and ocean.
Guy Stewart Callendar
The pause in the study of anthropogenic global warming continued for a surprisingly long time. After the first decade of the 20th century, it fell out of favor---and anyway seemed like a problem for thousands of years down the road. But there was one who thought differently.
Guy Stewart Callendar (1898-1964) was not a meteorologist. Or a climatologist. Or a physicist. In fact he was not a scientist of any kind! And a lot of his work was really flimsy. That was not all his fault---there was still no way of measuring carbon dioxide with the sort of pinpoint accuracy that we see today from Mauna Loa and other sites. But he was the only one who made any sort of impact at all during the long period of inactivity in greenhouse studies, so he is worth going over.
Callendar was the son of a prominent British physicist, Hugh Longbourne Callendar, and Victoria Mary Stewart. Hugh Longbourne Callendar specialized in thermodynamics, and held the lead physics chair at McGill University from 1893-1898. So Guy Stewart Callendar was born in Montreal. Hugh Callendar did make one innovation outside of thermodynamics---he developed and implemented the idea of using X-rays to inspect machine parts. He started with aircraft engines in World War I, helping to discover hidden defects and making engine manufacturing more efficient, by revealing how and where defects occurred---enabling manufacturing processes to be revised to lower defects and increase efficiency.
Back to Guy Callendar. As I said, he was not a scientist---although he stayed in the general field of his father. Guy was a power plant engineer, and he was a good one. From the 1920s on he developed methods to make energy production more efficient--which obviously required a good working knowledge of thermodynamics. He was also an amateur meteorologist. Well, not really. He didn't have a lot of training, although he read a lot of work in meteorology. But he never obtained a degree or took advanced coursework. And he was convinced the world was warming. And he was right about that. He was also convinced that mankind's CO2 emissions were responsible. About that, he was not right. At least not yet.
[It has to be said here that CO2 emissions, while rising rapidly from the 1870s on, were rising from a very low base, compared to today. By the 1920s and 1930s, CO2 emissions were just enough to have a very slight effect on climate, especially if sustained. However, they were not enough to account for the warming that took place from the 1880s to the 1930s, which must therefore have mostly resulted from natural causes.]
Whatever the causes, the warming was noticed by the 1920s and 1930s. Arctic ice shrank. Warming was most pronounced over the Arctic (Antarctica had no good weather records---expeditions kept meteorological diaries, but no permanent bases were established until the 1957-1958 International Geophysical Year, and there was only the testimony of the whalers who talked of "good ice years" and "bad ice years" and almost NEVER braved the Antarctic winter.
The warming was also most noted in continental interiors and less in the oceans--exactly what Arrhenius had predicted!
[This is also questionable. Callendar was basing this on the Dust Bowl drought of the 1930s, with the record heat waves in 1934 and 1936. Stalin's Soviet Union was not cooperating with other countries in releasing meteorological information. There was also political pressure on climate statistics there. Stalin and the Lysenko clique believed that the development of Siberia---the growth of cities and the leveling of the forests would result in a warmer climate. The point was made when meteorologists were executed when their records showed that the temperature had dropped from one year to the next. Temperature records therefore showed steady rises from year to year. This data was not released to the world, being a 'state secret'. Mongolia of course did not have regular meteorological observations that could be used to determine climate well. Republican China had a few places with reliable observations--Beijing, Shangghai, Hong Kong. But the Chinese did not have reliable climatic records for the interior.]
So on the basis of temperature records in Spitsbergen, Greendland, the Canadian Arctic, and the United States, Callendar concluded that anthropogenic global warming was occuring---and was occuring NOW (in the 1930s)
Callendar researched the levels of carbon dioxide in the atmosphere. The method for determining atmospheric CO2 concentrations was primitive, and not very accurate. What one did was get a dilute alkaline solution, and bubble air though it at a fixed temperature and rate and determined how much air flowed through before it neutralized the alkaline solution (CO2 being an acid compound). But this was fraught with difficulty. First of all, it assumed that all the CO2 in the air bubbling through the alkaline solution was interacting with it--what if some made it through? Second, observers did not always use the same alkaline compound---and we now know that CO2 reacts more easily with some basic compounds than others. Absorption of CO2, like all gases, varies according to temperature. The colder it is, the more easily the gas dissolves. And the apparatuses scientists used were not the same--each one tended to build his own apparatus (scientists being almost all male in those days--as far as I can tell, none of the pre-World War II observations were made by a woman)
There were also problems with local effects. The level of CO2 varies measurably between night and day. Downwind of herds of farm animals it was elevated. And observed CO2 levels in cities were very high--some of the observations in London during "pea soup" smogs when pollution was trapped under inversions were over 550 ppm! And those figures were probably correct. Greenwich Observatory reports figures over well over 500 ppm now during inversions.
Callendar was convinced that atmospheric CO2 was rising but it was almost impossible to prove. In fact, it was impossible, unless he made some arbitrary assumptions. First, he eliminated urban observations. Then he went over rural observations---and took out some downwind of large herds or in a couple of cases, power plants. After that, his decisions on what CO2 observations to include, and what not to include, seem mostly arbitrary. Callendar did include observations from ocean islands, like the Azores and Bermuda---and those were probably the best sites to observe what he was looking for. But his methodology was questionable. However in fairness, these primitive observations were all he had to work with.
Callendar wrote articles for science journals until the early 1960s, and here is his data reproduced from a late article. The observations he included when he made his presentation to the Royal Meteorological Society in 1938 are circled in the graph below.
As you can see, the observations Callendar chose do show a rising trend. And no one believed that the observations above 400 ppm were representative of the atmosphere of the Earth. But you can also see how there were a lot of observations Callendar excluded that seem like 'reasonable figures'.
Callendar was nervous as he addressed the Royal Meteorological Society. They listened politely. They did ask some questions about his choice of data. There was a little scattered applause when he finished.
And that was pretty much it. Callender published his work later in 1938 in the Journal of the Royal Meteorological Society. Callendar, G.S. (1938). "The Artificial Production of Carbon Dioxide and Its Influence on Climate." Quarterly J. Royal Meteorological Society 64: 223-40
After the questions about 'cherry picking' data and some correspondence with meteorologists, he omitted the graph above. His article contains some interesting statements, such as the one below:
"By fuel combustion, man has added about 150,000 million tons of carbon dioxide to the air during the past half century. The author estimates from the best available data that approximately three quarters of this has remained in the atmosphere."
n.b. At present we add that amount in 4 years.
Callendar believed that global warming would proceed at about 0.5C per century.
He believed that the concentration of CO2 in the atmosphere was 274 ppm before industrial activities began.
He believed that CO2 in 1936 was about 296 ppm.
Unlike Arrhenius, Callendar took into account economic growth. He believed that CO2 emissions were rising over time. So CO2 concentrations would go up faster and faster! But his estimates for the future seem quaint.
2000 AD 335 ppm
2100 AD 396 ppm (will probably be reached in 2012)
2200 AD 458 ppm (will probably be reached by 2060)
Callendar had some notions that were just plain wrong. We know that only about half of CO2 remains in the atmosphere. He also ignored the roles that convection and fronts have in redistributing heat in the atmosphere, and for this was roundly criticized.
But Callendar was also right in some ways. He believed that the oceans would not absorb all CO2 as it was emitted---pointing out correctly that if CO2 was absorbed so readily---then why was there any CO2 in the atmosphere at all? That caused many scientists some uneasiness--there must be some property of the ocean to resist absorbing CO2 to account for its presence.
Callendar was also right about the examination of CO2 absorption spectra and saturation. He argued that the behavior of CO2 infrared absorption in the cold dry upper levels of the atmosphere was not addressed by laboratory experiments at room temperature and pressure.
Callendar, G.S. (1941). "Infra-Red Absorption by Carbon Dioxide, with Special Reference to Atmospheric Radiation." Quarterly J. Royal Meteorological Society 67: 263-75.
However, most scientists believed Callendar was beating a dead horse there. Richard Russel, writing for the United States Department of Agriculture, pronounced that the absorption saturation was the "fatal flaw" in Callendar's argument. And that settled it for several years.
Russell, Richard J. (1941). "Climatic Change through the Ages." In Climate and Man. Yearbook of Agriculture, edited by United States Department of Agriculture. Washington, DC: US Govt. Printing Office.
For a non-meteorologist, Callendar's ideas did get a surprising amount of tolerance. Most meteorological textbooks of the 1940s and 1950s give him a short section, then rebuttals by meteorologists and physicists explaining why he was wrong. Callendar published many articles--but the general reaction of meteorologists at the time was to let him present his view, and address his ideas and answer his questions.
A short selection of more of his articles below:
Callendar, G.S. (1949). "Can Carbon Dioxide Influence Climate?" Weather 4: 310-14.
Callendar, G.S. (1958). "On the Amount of Carbon Dioxide in the Atmosphere." Tellus 10: 243-48.
Callendar, G.S. (1961). "Temperature Fluctuations and Trends over the Earth." Quarterly J. Royal Meteorological Society 87: 1-12.
Not everyone dismissed Callendar completely. Although the infrared radiation absorption saturation question was considered settled---the question of absorption of CO2 by the sea was not. It was well known that for every molecule of CO2 in the atmosphere, there were 50 in the sea. But then why didn't the sea absorb the 51st molecule?
Callendar thought that the oceans were stratified, that the surface layers did not mix readily with the deeper layers, and that the thin surface layer would be saturated quickly and not absorb more CO2. Harald Sverdrup, (1888-1957) perhaps the greatest oceanographer of the 20th century, and worthy of several blog entries himself, published his monumental work The Oceans: Their Physics, Chemistry and General Biology in 1942 and conclusively showed this was incorrect. The deep waters and surface of the oceans are exchanging readily.
So then what was keeping the oceans from absorbing all the CO2 in the atmosphere? No one was able to propose an acceptable mechanism for why the oceans would absorb CO2 until it reached 274 ppm and then stop.
Callendar lived long enough to see the first few years of the Mauna Loa readings taken by Charles Keeling. CO2 was accumulating in the atmosphere.
But that's for another blog entry.
Guy Stewart Callendar's picture:
Guy Stewart Callendar (1898-1964) was not a meteorologist. Or a climatologist. Or a physicist. In fact he was not a scientist of any kind! And a lot of his work was really flimsy. That was not all his fault---there was still no way of measuring carbon dioxide with the sort of pinpoint accuracy that we see today from Mauna Loa and other sites. But he was the only one who made any sort of impact at all during the long period of inactivity in greenhouse studies, so he is worth going over.
Callendar was the son of a prominent British physicist, Hugh Longbourne Callendar, and Victoria Mary Stewart. Hugh Longbourne Callendar specialized in thermodynamics, and held the lead physics chair at McGill University from 1893-1898. So Guy Stewart Callendar was born in Montreal. Hugh Callendar did make one innovation outside of thermodynamics---he developed and implemented the idea of using X-rays to inspect machine parts. He started with aircraft engines in World War I, helping to discover hidden defects and making engine manufacturing more efficient, by revealing how and where defects occurred---enabling manufacturing processes to be revised to lower defects and increase efficiency.
Back to Guy Callendar. As I said, he was not a scientist---although he stayed in the general field of his father. Guy was a power plant engineer, and he was a good one. From the 1920s on he developed methods to make energy production more efficient--which obviously required a good working knowledge of thermodynamics. He was also an amateur meteorologist. Well, not really. He didn't have a lot of training, although he read a lot of work in meteorology. But he never obtained a degree or took advanced coursework. And he was convinced the world was warming. And he was right about that. He was also convinced that mankind's CO2 emissions were responsible. About that, he was not right. At least not yet.
[It has to be said here that CO2 emissions, while rising rapidly from the 1870s on, were rising from a very low base, compared to today. By the 1920s and 1930s, CO2 emissions were just enough to have a very slight effect on climate, especially if sustained. However, they were not enough to account for the warming that took place from the 1880s to the 1930s, which must therefore have mostly resulted from natural causes.]
Whatever the causes, the warming was noticed by the 1920s and 1930s. Arctic ice shrank. Warming was most pronounced over the Arctic (Antarctica had no good weather records---expeditions kept meteorological diaries, but no permanent bases were established until the 1957-1958 International Geophysical Year, and there was only the testimony of the whalers who talked of "good ice years" and "bad ice years" and almost NEVER braved the Antarctic winter.
The warming was also most noted in continental interiors and less in the oceans--exactly what Arrhenius had predicted!
[This is also questionable. Callendar was basing this on the Dust Bowl drought of the 1930s, with the record heat waves in 1934 and 1936. Stalin's Soviet Union was not cooperating with other countries in releasing meteorological information. There was also political pressure on climate statistics there. Stalin and the Lysenko clique believed that the development of Siberia---the growth of cities and the leveling of the forests would result in a warmer climate. The point was made when meteorologists were executed when their records showed that the temperature had dropped from one year to the next. Temperature records therefore showed steady rises from year to year. This data was not released to the world, being a 'state secret'. Mongolia of course did not have regular meteorological observations that could be used to determine climate well. Republican China had a few places with reliable observations--Beijing, Shangghai, Hong Kong. But the Chinese did not have reliable climatic records for the interior.]
So on the basis of temperature records in Spitsbergen, Greendland, the Canadian Arctic, and the United States, Callendar concluded that anthropogenic global warming was occuring---and was occuring NOW (in the 1930s)
Callendar researched the levels of carbon dioxide in the atmosphere. The method for determining atmospheric CO2 concentrations was primitive, and not very accurate. What one did was get a dilute alkaline solution, and bubble air though it at a fixed temperature and rate and determined how much air flowed through before it neutralized the alkaline solution (CO2 being an acid compound). But this was fraught with difficulty. First of all, it assumed that all the CO2 in the air bubbling through the alkaline solution was interacting with it--what if some made it through? Second, observers did not always use the same alkaline compound---and we now know that CO2 reacts more easily with some basic compounds than others. Absorption of CO2, like all gases, varies according to temperature. The colder it is, the more easily the gas dissolves. And the apparatuses scientists used were not the same--each one tended to build his own apparatus (scientists being almost all male in those days--as far as I can tell, none of the pre-World War II observations were made by a woman)
There were also problems with local effects. The level of CO2 varies measurably between night and day. Downwind of herds of farm animals it was elevated. And observed CO2 levels in cities were very high--some of the observations in London during "pea soup" smogs when pollution was trapped under inversions were over 550 ppm! And those figures were probably correct. Greenwich Observatory reports figures over well over 500 ppm now during inversions.
Callendar was convinced that atmospheric CO2 was rising but it was almost impossible to prove. In fact, it was impossible, unless he made some arbitrary assumptions. First, he eliminated urban observations. Then he went over rural observations---and took out some downwind of large herds or in a couple of cases, power plants. After that, his decisions on what CO2 observations to include, and what not to include, seem mostly arbitrary. Callendar did include observations from ocean islands, like the Azores and Bermuda---and those were probably the best sites to observe what he was looking for. But his methodology was questionable. However in fairness, these primitive observations were all he had to work with.
Callendar wrote articles for science journals until the early 1960s, and here is his data reproduced from a late article. The observations he included when he made his presentation to the Royal Meteorological Society in 1938 are circled in the graph below.
As you can see, the observations Callendar chose do show a rising trend. And no one believed that the observations above 400 ppm were representative of the atmosphere of the Earth. But you can also see how there were a lot of observations Callendar excluded that seem like 'reasonable figures'.
Callendar was nervous as he addressed the Royal Meteorological Society. They listened politely. They did ask some questions about his choice of data. There was a little scattered applause when he finished.
And that was pretty much it. Callender published his work later in 1938 in the Journal of the Royal Meteorological Society. Callendar, G.S. (1938). "The Artificial Production of Carbon Dioxide and Its Influence on Climate." Quarterly J. Royal Meteorological Society 64: 223-40
After the questions about 'cherry picking' data and some correspondence with meteorologists, he omitted the graph above. His article contains some interesting statements, such as the one below:
"By fuel combustion, man has added about 150,000 million tons of carbon dioxide to the air during the past half century. The author estimates from the best available data that approximately three quarters of this has remained in the atmosphere."
n.b. At present we add that amount in 4 years.
Callendar believed that global warming would proceed at about 0.5C per century.
He believed that the concentration of CO2 in the atmosphere was 274 ppm before industrial activities began.
He believed that CO2 in 1936 was about 296 ppm.
Unlike Arrhenius, Callendar took into account economic growth. He believed that CO2 emissions were rising over time. So CO2 concentrations would go up faster and faster! But his estimates for the future seem quaint.
2000 AD 335 ppm
2100 AD 396 ppm (will probably be reached in 2012)
2200 AD 458 ppm (will probably be reached by 2060)
Callendar had some notions that were just plain wrong. We know that only about half of CO2 remains in the atmosphere. He also ignored the roles that convection and fronts have in redistributing heat in the atmosphere, and for this was roundly criticized.
But Callendar was also right in some ways. He believed that the oceans would not absorb all CO2 as it was emitted---pointing out correctly that if CO2 was absorbed so readily---then why was there any CO2 in the atmosphere at all? That caused many scientists some uneasiness--there must be some property of the ocean to resist absorbing CO2 to account for its presence.
Callendar was also right about the examination of CO2 absorption spectra and saturation. He argued that the behavior of CO2 infrared absorption in the cold dry upper levels of the atmosphere was not addressed by laboratory experiments at room temperature and pressure.
Callendar, G.S. (1941). "Infra-Red Absorption by Carbon Dioxide, with Special Reference to Atmospheric Radiation." Quarterly J. Royal Meteorological Society 67: 263-75.
However, most scientists believed Callendar was beating a dead horse there. Richard Russel, writing for the United States Department of Agriculture, pronounced that the absorption saturation was the "fatal flaw" in Callendar's argument. And that settled it for several years.
Russell, Richard J. (1941). "Climatic Change through the Ages." In Climate and Man. Yearbook of Agriculture, edited by United States Department of Agriculture. Washington, DC: US Govt. Printing Office.
For a non-meteorologist, Callendar's ideas did get a surprising amount of tolerance. Most meteorological textbooks of the 1940s and 1950s give him a short section, then rebuttals by meteorologists and physicists explaining why he was wrong. Callendar published many articles--but the general reaction of meteorologists at the time was to let him present his view, and address his ideas and answer his questions.
A short selection of more of his articles below:
Callendar, G.S. (1949). "Can Carbon Dioxide Influence Climate?" Weather 4: 310-14.
Callendar, G.S. (1958). "On the Amount of Carbon Dioxide in the Atmosphere." Tellus 10: 243-48.
Callendar, G.S. (1961). "Temperature Fluctuations and Trends over the Earth." Quarterly J. Royal Meteorological Society 87: 1-12.
Not everyone dismissed Callendar completely. Although the infrared radiation absorption saturation question was considered settled---the question of absorption of CO2 by the sea was not. It was well known that for every molecule of CO2 in the atmosphere, there were 50 in the sea. But then why didn't the sea absorb the 51st molecule?
Callendar thought that the oceans were stratified, that the surface layers did not mix readily with the deeper layers, and that the thin surface layer would be saturated quickly and not absorb more CO2. Harald Sverdrup, (1888-1957) perhaps the greatest oceanographer of the 20th century, and worthy of several blog entries himself, published his monumental work The Oceans: Their Physics, Chemistry and General Biology in 1942 and conclusively showed this was incorrect. The deep waters and surface of the oceans are exchanging readily.
So then what was keeping the oceans from absorbing all the CO2 in the atmosphere? No one was able to propose an acceptable mechanism for why the oceans would absorb CO2 until it reached 274 ppm and then stop.
Callendar lived long enough to see the first few years of the Mauna Loa readings taken by Charles Keeling. CO2 was accumulating in the atmosphere.
But that's for another blog entry.
Guy Stewart Callendar's picture:
Wednesday, March 9, 2011
Modeling Weather Part 2
Sometimes scientists and engineers have the misfortune of being born too early. Not just a decade or two early, but generations. Charles Babbage is one. Lewis Fry Richardson
(1881-1953) is another.
Lewis Fry Richardson was born to a Quaker family and was a life-long pacifist. His parents, who owned a leather goods factory, were well off, and saw to it that he got a first rate education. In 1898 he attended the Durham College of Science, where he took courses in mathematical physics, chemistry, botany, and zoology, excelling in them all. He was interested in many sciences and remained so for the rest of his life. In 1900 he received a scholarship to Kings College, Cambridge, where he took the Natural Sciences Tripos, a course of study for people interested in many sciences. He graduated first in his class. His refusal to specialize prevented him from receiving a doctorate at that time, although ultimately he did receive a doctorate in mathematical psychology from the University of London in 1928.
In 1909 he married Dorothy Gannet, to whom he stayed married for the rest of his life. They were happy together, but also had sadness. They experienced 3 miscarriages, despite both being healthy. Only much later did they realize they had incompatible blood types---he was RH positive and she was RH negative. They adopted two sons and a daughter between 1920 and 1927.
Richardson's career reflects his eclectic interests:
* National Physical Laboratory (1903–1904)
* University College Aberystwyth (1905–1906)
* chemist, National Peat Industries (1906–1907)
* National Physical Laboratory (1907–1909)
* manager of the physical and chemical laboratory, Sunbeam Lamp Company (1909–1912)
* Manchester College of Technology (1912–1913)
* Meteorological Office - as superintendent of Eskdalemuir Observatory (1913–1916)
* Friends Ambulance Unit in France (1916–1919)
* Meteorological Office at Benson, Oxfordshire (1919–1920)
* Head of the Physics Department at Westminster Training College (1920–1929)
* Principal, Paisley Technical College, now part of the University of the West of Scotland (1929–1940)
In 1926, he was elected to the Fellowship of the Royal Society
Richardson made original contributions in many fields, but today is known chiefly for his meteorological insights and his research into fractals (a branch of mathematics not named for more than 20 years after he died.
A brief description of his work in fractals is worthy of mention.
A lifelong pacifist (he did not fight in World War I, and was an ambulance driver) he wondered if there was a mathematical basis for countries going to war with each other. In his research, he came across widely varying figures for the length of borders between nations. For instance, 380 km or 449 km between The Netherlands and Belgium. 987 km or 1,214 km between Spain and Portugal. How could this be?
He dropped his research into the mathematical causes of war between countries for a while, and wondered about the coastline of Britain.
He measured the coastline of Britain using a 200 km ruler:
Then with a 100 km ruler:
Then with a 50 km ruler:
Notice how the length of the coastline increases every time one uses a finer unit to measure! Richardson proved that there was no final measurement of the length of the coastline that could be made. For an irregular object, measuring its perimeter depends on the length of the unit you are using to make the measurement. The smaller the unit of measurement, the greater the total perimeter will be. This work was ignored for more than 40 years, but when Benoit Mandelbrot ran across it in the 1960s, it became the inspiration for his work How Long is the Coastline of Britain (1967), which was the beginning of his development of fractal geometry.
Richardson also made original contributions to numerical analysis, developing new equations for the convergence of a sequence. Details of his mathematical work in this field are here.
Richardson also created a new iterative method for solving linear equations, which is now known as the Richardson Iteration Method
Richardson's mathematical contributions to meteorology began with his investigations into the mathematics of turbulence. He developed a way of calculating the ratio of potential energy to kinetic energy:
in which g is the acceleration due to gravity, h a representative vertical lengthscale, and u a representative speed.
When considering flows in which density differences are small it is common to use the reduced gravity g' and the relevant parameter is the densimetric Richardson number.
Richardson was always interested in meteorology, and through his work as an javascript:void(0)ambulance driver in World War I, he made the first computational weather forecast. He collected observation records over central Europe for an arbitrary day he chose, May 20, 1910, with measurements of atmospheric characteristics and vectors according to the new Bjerknes paradigm and tried to calculate what the weather would be in 3 days time. It was very difficult and intricate computational work, and he had to deal with interruptions not typical for today's research scientists. In April 1917 when the German army was on a local offensive, his ambulance was shelled and he had to get out of the wreckage and bury his notebooks in a metal container in a local churchyard. Then the German army took over and he had to wait for months to go back when the village was retaken. Fortunately his work survived.
His computations were discouraging. His first 'retrocast' showed impossible barometric pressure rises of more than 4.3 inches (135 mb) over central Europe, and he was discouraged. He also had a good mathematical intuition---that small variances in initial conditions would have big impacts on the future weather---a step towards chaos theory (which came from Edward Norton Lorenz in the 1960s and 1970s, also a meteorologist)
Richarson composed a little poem:
Big whirls have little whirls
That feed on their velocity
And little whirls have lesser whirls
And so on to viscosity
The error was not identified until 2007. The error had to do with a smoothing method for interpolating between data points--Richardson had known about this method, but being shell-shocked had just forgot! Without the error, his forecast was basically correct. Three days out.
But he never knew that.
Richardson conceived of a way to forecast weather accurately, worldwide, in advance. He imagined 60,000 mathematicians working at any one time on the weather, 300,000 in shifts. With data from aircraft, a worldwide network of balloons, station observations, ships, buoys, all transmitted to computers to work out the data.
But not computers the way we think of them. Computers were the people doing the computing! And I have to imagine it would be tedious work! It's like something out of a John Varley novella. At this moment Overdrawn at the Memory Bank is replaying in my head ;)
Richardson wrote all this out in a monumental work that reads as much like science fiction as science, Weather Prediction by Numerical Processes (1922). It is well worth a read in the original.
Here is a passage from the book:
“After so much hard reasoning, may one play with a fantasy? Imagine a large hall like a theatre, except that the circles and galleries go right round through the space usually occupied by the stage. The walls of this chamber are painted to form a map of the globe. The ceiling represents the north polar regions, England is in the gallery, the tropics in the upper circle, Australia on the dress circle and the Antarctic in the pit.
A myriad computers are at work upon the weather of the part of the map where each sits, but each computer attends only to one equation or part of an equation. The work of each region is coordinated by an official of higher rank. Numerous little "night signs" display the instantaneous values so that neighbouring computers can read them. Each number is thus displayed in three adjacent zones so as to maintain communication to the North and South on the map.
From the floor of the pit a tall pillar rises to half the height of the hall. It carries a large pulpit on its top. In this sits the man in charge of the whole theatre; he is surrounded by several assistants and messengers. One of his duties is to maintain a uniform speed of progress in all parts of the globe. In this respect he is like the conductor of an orchestra in which the instruments are slide-rules and calculating machines. But instead of waving a baton he turns a beam of rosy light upon any region that is running ahead of the rest, and a beam of blue light upon those who are behindhand.
Four senior clerks in the central pulpit are collecting the future weather as fast as it is being computed, and despatching it by pneumatic carrier to a quiet room. There it will be coded and telephoned to the radio transmitting station. Messengers carry piles of used computing forms down to a storehouse in the cellar.
In a neighbouring building there is a research department, where they invent improvements. But these is much experimenting on a small scale before any change is made in the complex routine of the computing theatre. In a basement an enthusiast is observing eddies in the liquid lining of a huge spinning bowl, but so far the arithmetic proves the better way. In another building are all the usual financial, correspondence and administrative offices. Outside are playing fields, houses, mountains and lakes, for it was thought that those who compute the weather should breathe of it freely.”
Richardson of course knew that the idea of 300,000 mathematicians employed for computing the weather was a fantasy. But it would work!
Richardson thought he would never live to see it: "Perhaps some day in the dim future it will be possible to advance the computations faster than the weather
advances.....But that is a dream."
Richardson discussed the way the meteorologists of his time made forecasts---they studied the maps they created, remembered past occasions when the maps were similar, and made forecasts based on what happened next during prior occasions when the weather maps were similar. Richardson took a dim view of this.
"The forecast is based on the supposition that what the atmosphere
did then, it will do again now.....The past history of the atmosphere is used, so to speak, as a full-scale working model of its present self"
Richardson compared this to astronomical predictions of the positions of the planets and stars:
"The Nautical Almanac, that marvel of accurate forecasting, is not based on the principle that astronomical history repeats itself in the aggregate. It would be safe to say that a particular disposition of stars, planets and satellites never occurs twice. Why then should we expect a present weather map to be exactly represented in a catalogue of past weather?"
Another quote from Richardson:
"The scheme is complicated because the atmosphere is complicated."
The 'scheme' referring to hundreds of thousands of mathematicians working on the weather.
Inexplicably, wikipedia does not have an article on "Weather Prediction by Numerical Process". Fortunately, google books does have it. Credit to John for finding it when I failed!
Reviews of "Weather Prediction by Numerical Process" were quite positive, although many thought the idea of using computational power--either through hundreds of thousands of mathematicians or by mechanical calculators to be a fantasy. There was also the problem of his forecast for May 20, 1910--with the wildly high barometric pressure. The book didn't sell well, and dropped out of sight. I came across it on the stacks of the GA Tech library in 1993 and read it---it was fascinating to me.
Richardson left the Meteorological Office in 1920 when it was made a part of the Air Ministry, which later became part of the Ministry of Defence. As "Weather Prediction by Numerical Process" was being published, Richardson discovered that his work on meteorology and atmospheric motion was being used to predict how to deploy mustard gas and chlorine--gas warfare. Richardson, as a life-long pacifist, was outraged. He removed all his work and notes from his office, destroyed them and then resigned. That caused a lot of controversy, but it did not stop him from being elected as a fellow of the Royal Society in 1926.
Disillusioned with the military appropriation of his work for gas warfare, he became interested in psychology (recall his doctorate) and the mathematics of violence. He worked in that field for the rest of his life, although maintaining himself in other disciplines. In 1949 Richardson published "Arms and Insecurity" in which he demonstrated that the length of two countries' common border was very strongly correlated with the risk of their going to war, among other factors. In 1950, he published "Statistics of Deadly Quarrels" in which he researched every conflict from 1815 to 1945 and discovered that the size of wars' death tolls varied by a logrithmic scale, very close to a base ten logarithmic scale. There were many more small conflicts than large ones, and the death tolls followed a Poisson distribution very closely. Richardson also determined this was true for gangland murders in Chicago and Shanghai---there were very many cases of one or two murders, 3 or 4 being less common and incidents like the St. Valentine's Day Massacre being very rare.
Richardson was not quite as out of time as Charles Babbage. Babbage never saw a modern computer. But Richardson was still alive when ENIAC made the first weather prediction in 1950. Richardson called it "an enormous advance".
A truly excellent summation of the history of numerical weather prediction is The origins of computer weather prediction and climate modeling by Peter Lynch. It is well worth a read!
(1881-1953) is another.
Lewis Fry Richardson was born to a Quaker family and was a life-long pacifist. His parents, who owned a leather goods factory, were well off, and saw to it that he got a first rate education. In 1898 he attended the Durham College of Science, where he took courses in mathematical physics, chemistry, botany, and zoology, excelling in them all. He was interested in many sciences and remained so for the rest of his life. In 1900 he received a scholarship to Kings College, Cambridge, where he took the Natural Sciences Tripos, a course of study for people interested in many sciences. He graduated first in his class. His refusal to specialize prevented him from receiving a doctorate at that time, although ultimately he did receive a doctorate in mathematical psychology from the University of London in 1928.
In 1909 he married Dorothy Gannet, to whom he stayed married for the rest of his life. They were happy together, but also had sadness. They experienced 3 miscarriages, despite both being healthy. Only much later did they realize they had incompatible blood types---he was RH positive and she was RH negative. They adopted two sons and a daughter between 1920 and 1927.
Richardson's career reflects his eclectic interests:
* National Physical Laboratory (1903–1904)
* University College Aberystwyth (1905–1906)
* chemist, National Peat Industries (1906–1907)
* National Physical Laboratory (1907–1909)
* manager of the physical and chemical laboratory, Sunbeam Lamp Company (1909–1912)
* Manchester College of Technology (1912–1913)
* Meteorological Office - as superintendent of Eskdalemuir Observatory (1913–1916)
* Friends Ambulance Unit in France (1916–1919)
* Meteorological Office at Benson, Oxfordshire (1919–1920)
* Head of the Physics Department at Westminster Training College (1920–1929)
* Principal, Paisley Technical College, now part of the University of the West of Scotland (1929–1940)
In 1926, he was elected to the Fellowship of the Royal Society
Richardson made original contributions in many fields, but today is known chiefly for his meteorological insights and his research into fractals (a branch of mathematics not named for more than 20 years after he died.
A brief description of his work in fractals is worthy of mention.
A lifelong pacifist (he did not fight in World War I, and was an ambulance driver) he wondered if there was a mathematical basis for countries going to war with each other. In his research, he came across widely varying figures for the length of borders between nations. For instance, 380 km or 449 km between The Netherlands and Belgium. 987 km or 1,214 km between Spain and Portugal. How could this be?
He dropped his research into the mathematical causes of war between countries for a while, and wondered about the coastline of Britain.
He measured the coastline of Britain using a 200 km ruler:
Then with a 100 km ruler:
Then with a 50 km ruler:
Notice how the length of the coastline increases every time one uses a finer unit to measure! Richardson proved that there was no final measurement of the length of the coastline that could be made. For an irregular object, measuring its perimeter depends on the length of the unit you are using to make the measurement. The smaller the unit of measurement, the greater the total perimeter will be. This work was ignored for more than 40 years, but when Benoit Mandelbrot ran across it in the 1960s, it became the inspiration for his work How Long is the Coastline of Britain (1967), which was the beginning of his development of fractal geometry.
Richardson also made original contributions to numerical analysis, developing new equations for the convergence of a sequence. Details of his mathematical work in this field are here.
Richardson also created a new iterative method for solving linear equations, which is now known as the Richardson Iteration Method
Richardson's mathematical contributions to meteorology began with his investigations into the mathematics of turbulence. He developed a way of calculating the ratio of potential energy to kinetic energy:
in which g is the acceleration due to gravity, h a representative vertical lengthscale, and u a representative speed.
When considering flows in which density differences are small it is common to use the reduced gravity g' and the relevant parameter is the densimetric Richardson number.
Richardson was always interested in meteorology, and through his work as an javascript:void(0)ambulance driver in World War I, he made the first computational weather forecast. He collected observation records over central Europe for an arbitrary day he chose, May 20, 1910, with measurements of atmospheric characteristics and vectors according to the new Bjerknes paradigm and tried to calculate what the weather would be in 3 days time. It was very difficult and intricate computational work, and he had to deal with interruptions not typical for today's research scientists. In April 1917 when the German army was on a local offensive, his ambulance was shelled and he had to get out of the wreckage and bury his notebooks in a metal container in a local churchyard. Then the German army took over and he had to wait for months to go back when the village was retaken. Fortunately his work survived.
His computations were discouraging. His first 'retrocast' showed impossible barometric pressure rises of more than 4.3 inches (135 mb) over central Europe, and he was discouraged. He also had a good mathematical intuition---that small variances in initial conditions would have big impacts on the future weather---a step towards chaos theory (which came from Edward Norton Lorenz in the 1960s and 1970s, also a meteorologist)
Richarson composed a little poem:
Big whirls have little whirls
That feed on their velocity
And little whirls have lesser whirls
And so on to viscosity
The error was not identified until 2007. The error had to do with a smoothing method for interpolating between data points--Richardson had known about this method, but being shell-shocked had just forgot! Without the error, his forecast was basically correct. Three days out.
But he never knew that.
Richardson conceived of a way to forecast weather accurately, worldwide, in advance. He imagined 60,000 mathematicians working at any one time on the weather, 300,000 in shifts. With data from aircraft, a worldwide network of balloons, station observations, ships, buoys, all transmitted to computers to work out the data.
But not computers the way we think of them. Computers were the people doing the computing! And I have to imagine it would be tedious work! It's like something out of a John Varley novella. At this moment Overdrawn at the Memory Bank is replaying in my head ;)
Richardson wrote all this out in a monumental work that reads as much like science fiction as science, Weather Prediction by Numerical Processes (1922). It is well worth a read in the original.
Here is a passage from the book:
“After so much hard reasoning, may one play with a fantasy? Imagine a large hall like a theatre, except that the circles and galleries go right round through the space usually occupied by the stage. The walls of this chamber are painted to form a map of the globe. The ceiling represents the north polar regions, England is in the gallery, the tropics in the upper circle, Australia on the dress circle and the Antarctic in the pit.
A myriad computers are at work upon the weather of the part of the map where each sits, but each computer attends only to one equation or part of an equation. The work of each region is coordinated by an official of higher rank. Numerous little "night signs" display the instantaneous values so that neighbouring computers can read them. Each number is thus displayed in three adjacent zones so as to maintain communication to the North and South on the map.
From the floor of the pit a tall pillar rises to half the height of the hall. It carries a large pulpit on its top. In this sits the man in charge of the whole theatre; he is surrounded by several assistants and messengers. One of his duties is to maintain a uniform speed of progress in all parts of the globe. In this respect he is like the conductor of an orchestra in which the instruments are slide-rules and calculating machines. But instead of waving a baton he turns a beam of rosy light upon any region that is running ahead of the rest, and a beam of blue light upon those who are behindhand.
Four senior clerks in the central pulpit are collecting the future weather as fast as it is being computed, and despatching it by pneumatic carrier to a quiet room. There it will be coded and telephoned to the radio transmitting station. Messengers carry piles of used computing forms down to a storehouse in the cellar.
In a neighbouring building there is a research department, where they invent improvements. But these is much experimenting on a small scale before any change is made in the complex routine of the computing theatre. In a basement an enthusiast is observing eddies in the liquid lining of a huge spinning bowl, but so far the arithmetic proves the better way. In another building are all the usual financial, correspondence and administrative offices. Outside are playing fields, houses, mountains and lakes, for it was thought that those who compute the weather should breathe of it freely.”
Richardson of course knew that the idea of 300,000 mathematicians employed for computing the weather was a fantasy. But it would work!
Richardson thought he would never live to see it: "Perhaps some day in the dim future it will be possible to advance the computations faster than the weather
advances.....But that is a dream."
Richardson discussed the way the meteorologists of his time made forecasts---they studied the maps they created, remembered past occasions when the maps were similar, and made forecasts based on what happened next during prior occasions when the weather maps were similar. Richardson took a dim view of this.
"The forecast is based on the supposition that what the atmosphere
did then, it will do again now.....The past history of the atmosphere is used, so to speak, as a full-scale working model of its present self"
Richardson compared this to astronomical predictions of the positions of the planets and stars:
"The Nautical Almanac, that marvel of accurate forecasting, is not based on the principle that astronomical history repeats itself in the aggregate. It would be safe to say that a particular disposition of stars, planets and satellites never occurs twice. Why then should we expect a present weather map to be exactly represented in a catalogue of past weather?"
Another quote from Richardson:
"The scheme is complicated because the atmosphere is complicated."
The 'scheme' referring to hundreds of thousands of mathematicians working on the weather.
Inexplicably, wikipedia does not have an article on "Weather Prediction by Numerical Process". Fortunately, google books does have it. Credit to John for finding it when I failed!
Reviews of "Weather Prediction by Numerical Process" were quite positive, although many thought the idea of using computational power--either through hundreds of thousands of mathematicians or by mechanical calculators to be a fantasy. There was also the problem of his forecast for May 20, 1910--with the wildly high barometric pressure. The book didn't sell well, and dropped out of sight. I came across it on the stacks of the GA Tech library in 1993 and read it---it was fascinating to me.
Richardson left the Meteorological Office in 1920 when it was made a part of the Air Ministry, which later became part of the Ministry of Defence. As "Weather Prediction by Numerical Process" was being published, Richardson discovered that his work on meteorology and atmospheric motion was being used to predict how to deploy mustard gas and chlorine--gas warfare. Richardson, as a life-long pacifist, was outraged. He removed all his work and notes from his office, destroyed them and then resigned. That caused a lot of controversy, but it did not stop him from being elected as a fellow of the Royal Society in 1926.
Disillusioned with the military appropriation of his work for gas warfare, he became interested in psychology (recall his doctorate) and the mathematics of violence. He worked in that field for the rest of his life, although maintaining himself in other disciplines. In 1949 Richardson published "Arms and Insecurity" in which he demonstrated that the length of two countries' common border was very strongly correlated with the risk of their going to war, among other factors. In 1950, he published "Statistics of Deadly Quarrels" in which he researched every conflict from 1815 to 1945 and discovered that the size of wars' death tolls varied by a logrithmic scale, very close to a base ten logarithmic scale. There were many more small conflicts than large ones, and the death tolls followed a Poisson distribution very closely. Richardson also determined this was true for gangland murders in Chicago and Shanghai---there were very many cases of one or two murders, 3 or 4 being less common and incidents like the St. Valentine's Day Massacre being very rare.
Richardson was not quite as out of time as Charles Babbage. Babbage never saw a modern computer. But Richardson was still alive when ENIAC made the first weather prediction in 1950. Richardson called it "an enormous advance".
A truly excellent summation of the history of numerical weather prediction is The origins of computer weather prediction and climate modeling by Peter Lynch. It is well worth a read!
Saturday, March 5, 2011
Modeling Weather Part 1
Again, this post is not strictly related to global warming. This post is about the beginning of weather modeling. Not many are aware that many of the principles and procedures of weather modeling began more than 20 years before the invention of computers.
Vilhelm Bjerknes (1862-1951) was a groudbreaking meteorologist who in 1895 developed primitive equations used to approximate atmospheric flow--and are still used in modern weather prediction models.
The forces involved are gravity, the pressure gradient, and viscous friction.
The equation terms:
* u is the zonal velocity (velocity in the east/west direction tangent to the sphere)
* v is the meridional velocity (velocity in the north/south direction tangent to the sphere)
* ω is the vertical velocity in isobaric coordinates
* T is the temperature
* Φ is the geopotential
* f is the term corresponding to the Coriolis force, and is equal to 2Ωsin(φ), where Ω is the angular rotation rate of the Earth (2π / 24 radians per sideral hour), and φ is the latitude
* R is the gas constant
* p is the pressure
* cp is the specific heat on a constant pressure surface
* J is the heat flow per unit time per unit mass
* W is the precipitable water
* Π is the Exner function
* θ is the potential temperature
The pressure gradient force causes an acceleration forcing air from regions of high pressure to regions of low pressure. Mathematically, this can be written as:
The gravitational force accelerates objects at approximately 9.81 m/s2 directly towards the center of the Earth. The force due to viscous friction can be approximated as:
Therefore, to complete the system of equations and obtain 6 equations and 6 variables:
Using Newton's second law, these forces (referenced in the equations above as the accelerations due to these forces) may be summed to produce an equation of motion that describes this system. This equation can be written in the form:
p = ρRT.
Bjerknes's work inspired Wagn Walfrid Eckman (1874-1954), a Swedish oceanographer and Carl-Gustaf Arvid Rossby in their work on the fluid motions of the oceans and the atmosphere, and laid the groundwork for the discovery of the Eckman Spiral and Rossby Waves.
The Eckman Spiral is one of the most counterintuitive discoveries in oceanography. Imagine a permanent large high pressure center over the middle of an ocean, like the Bermuda-Azores high. The winds circle around counterclockwise. But the effects of the Coriolis effect and friction cause the water right at the surface to move a little bit to the right of the wind motion. The water right under the topmost layer moves a little to the right of the topmost layer, and loses a little force through friction. Each layer down moves a little to the right, and loses a little force due to friction. Eckman was able to sum this up and discovered that the sum of all these currents is a tremendous inwards flow towards the center of the high pressure.
Oceanographers and meteorologists, when they thought about it at all, just assumed that the water level under a large permanent high pressure system was several inches lower than the surrounding water. Instead, Eckman showed it is several feet higher! This was so counterintuitive that for decades oceanographers had difficulty believing it was true, although it did explain why noreasters bring such high tides to the Atlantic coast. The sum of the force of the currents is 90 degrees to the right of the wind---a prolonged northeast wind will drive water to the northwest onto land. And Eckman's work was finally confirmed by satellite observations of sea level.
Eckman's spiral illustrated:
Ocean currents changing directions at an angle to the surface winds because of Coriolis effect
1. Wind
2. force from above
3. Effective direction of the current flow
4. Coriolis effect
Rossby waves are perturbations in the atmosphere that result from the variation in the strength of the Coriolis effect with latitude. The mathematical equations are considerably more numerous and complex than the primitive equations I posted, so I will omit those from the entry. Rossby waves are key to the transport of cooler air equatorward and warm air poleward.
Rossby waves are illustrated below, showing in this example how cold air can be drawn equatorward:
Back to Vilhelm Bjerknes. By 1913, he had come up with 7 variables that are capable of describing the characteristics and behavior of the atmosphere. These are:
The characteristics:
1. Pressure
2. Temperature
3. Density
4. Water vapor content
The vectors (wind)
5. East
6. North
7. Up
For the wind vectors, negative numbers would be used for the wind moving from a westerly direction, a southerly direction, or a downward direction (subsidence).
Thanks to Bjerknes, Rossby, and Eckman (a LOT of other meteorologists, physicists, and mathematicians also made contributions, but these are the giants) we had the mathematical tools to predict weather. And with some modifications, climate. There were some big problems though. How could we ever get enough data to make meaningful use of the equations. And even if we had enough data, how could we ever solve the equations fast enough to make timely forecasts?
100 years ago these problems seemed insurmountable. But Lewis Fry Richardson was about to try. And in the next entry, I'll tell you about him.
Vilhelm Bjerknes (1862-1951) was a groudbreaking meteorologist who in 1895 developed primitive equations used to approximate atmospheric flow--and are still used in modern weather prediction models.
The forces involved are gravity, the pressure gradient, and viscous friction.
The equation terms:
* u is the zonal velocity (velocity in the east/west direction tangent to the sphere)
* v is the meridional velocity (velocity in the north/south direction tangent to the sphere)
* ω is the vertical velocity in isobaric coordinates
* T is the temperature
* Φ is the geopotential
* f is the term corresponding to the Coriolis force, and is equal to 2Ωsin(φ), where Ω is the angular rotation rate of the Earth (2π / 24 radians per sideral hour), and φ is the latitude
* R is the gas constant
* p is the pressure
* cp is the specific heat on a constant pressure surface
* J is the heat flow per unit time per unit mass
* W is the precipitable water
* Π is the Exner function
* θ is the potential temperature
The pressure gradient force causes an acceleration forcing air from regions of high pressure to regions of low pressure. Mathematically, this can be written as:
The gravitational force accelerates objects at approximately 9.81 m/s2 directly towards the center of the Earth. The force due to viscous friction can be approximated as:
Therefore, to complete the system of equations and obtain 6 equations and 6 variables:
Using Newton's second law, these forces (referenced in the equations above as the accelerations due to these forces) may be summed to produce an equation of motion that describes this system. This equation can be written in the form:
p = ρRT.
Bjerknes's work inspired Wagn Walfrid Eckman (1874-1954), a Swedish oceanographer and Carl-Gustaf Arvid Rossby in their work on the fluid motions of the oceans and the atmosphere, and laid the groundwork for the discovery of the Eckman Spiral and Rossby Waves.
The Eckman Spiral is one of the most counterintuitive discoveries in oceanography. Imagine a permanent large high pressure center over the middle of an ocean, like the Bermuda-Azores high. The winds circle around counterclockwise. But the effects of the Coriolis effect and friction cause the water right at the surface to move a little bit to the right of the wind motion. The water right under the topmost layer moves a little to the right of the topmost layer, and loses a little force through friction. Each layer down moves a little to the right, and loses a little force due to friction. Eckman was able to sum this up and discovered that the sum of all these currents is a tremendous inwards flow towards the center of the high pressure.
Oceanographers and meteorologists, when they thought about it at all, just assumed that the water level under a large permanent high pressure system was several inches lower than the surrounding water. Instead, Eckman showed it is several feet higher! This was so counterintuitive that for decades oceanographers had difficulty believing it was true, although it did explain why noreasters bring such high tides to the Atlantic coast. The sum of the force of the currents is 90 degrees to the right of the wind---a prolonged northeast wind will drive water to the northwest onto land. And Eckman's work was finally confirmed by satellite observations of sea level.
Eckman's spiral illustrated:
Ocean currents changing directions at an angle to the surface winds because of Coriolis effect
1. Wind
2. force from above
3. Effective direction of the current flow
4. Coriolis effect
Rossby waves are perturbations in the atmosphere that result from the variation in the strength of the Coriolis effect with latitude. The mathematical equations are considerably more numerous and complex than the primitive equations I posted, so I will omit those from the entry. Rossby waves are key to the transport of cooler air equatorward and warm air poleward.
Rossby waves are illustrated below, showing in this example how cold air can be drawn equatorward:
Back to Vilhelm Bjerknes. By 1913, he had come up with 7 variables that are capable of describing the characteristics and behavior of the atmosphere. These are:
The characteristics:
1. Pressure
2. Temperature
3. Density
4. Water vapor content
The vectors (wind)
5. East
6. North
7. Up
For the wind vectors, negative numbers would be used for the wind moving from a westerly direction, a southerly direction, or a downward direction (subsidence).
Thanks to Bjerknes, Rossby, and Eckman (a LOT of other meteorologists, physicists, and mathematicians also made contributions, but these are the giants) we had the mathematical tools to predict weather. And with some modifications, climate. There were some big problems though. How could we ever get enough data to make meaningful use of the equations. And even if we had enough data, how could we ever solve the equations fast enough to make timely forecasts?
100 years ago these problems seemed insurmountable. But Lewis Fry Richardson was about to try. And in the next entry, I'll tell you about him.
Subscribe to:
Posts (Atom)